Effects after stopping Ozempic can include changes in appetite, blood glucose control, and body weight as the medication's influence gradually diminishes. Ozempic (semaglutide) is a GLP-1 receptor agonist licensed in the UK for type 2 diabetes management. When discontinued, its pharmacological effects—including appetite suppression, delayed gastric emptying, and enhanced insulin secretion—reverse over approximately five weeks as the drug is eliminated from the body. Understanding what to expect after stopping Ozempic helps patients prepare for the transition and implement appropriate strategies to maintain metabolic health and glycaemic control.
Summary: After stopping Ozempic, patients typically experience a gradual return of appetite, potential blood glucose elevation, and possible weight regain over several weeks to months as the medication's effects diminish.
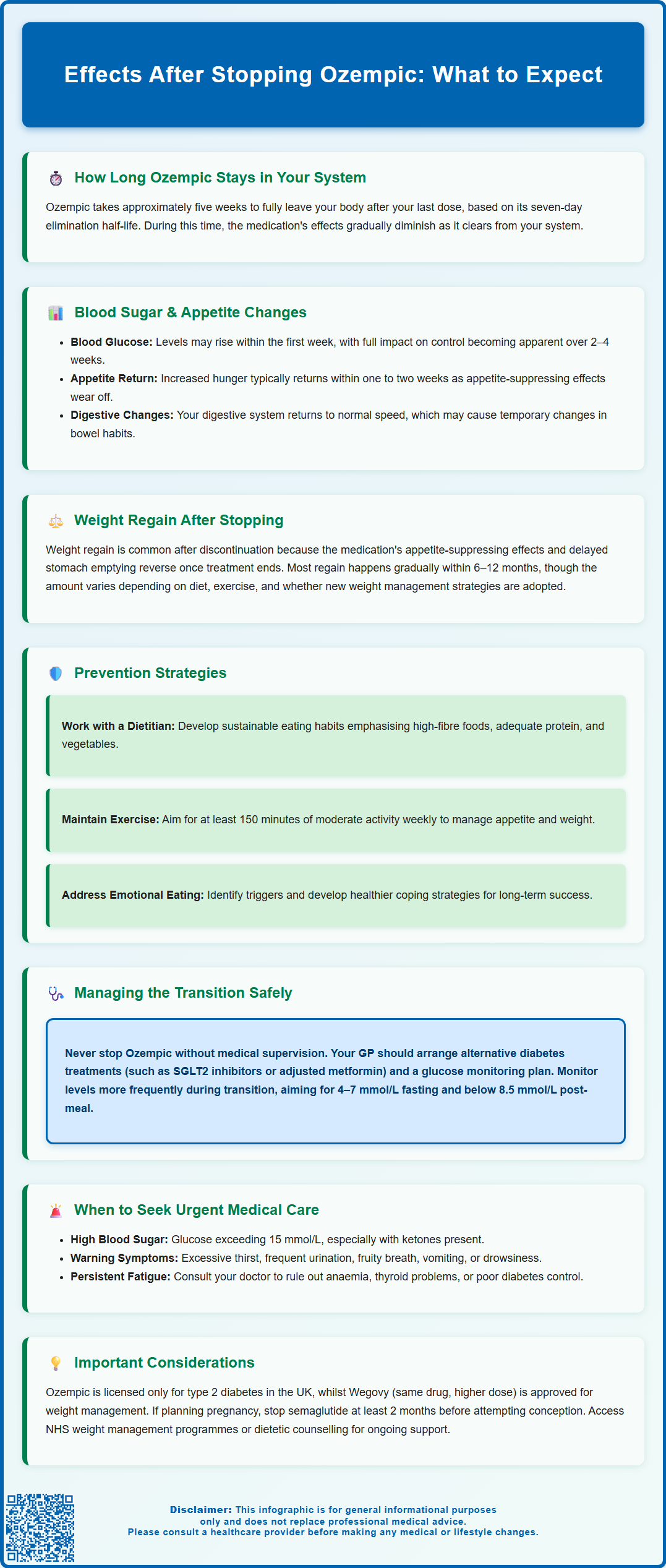
- Semaglutide has a one-week half-life and takes approximately five weeks to be substantially eliminated from the body after discontinuation.
- Appetite suppression diminishes within one to two weeks, often resulting in increased hunger as central nervous system effects reverse.
- Blood glucose levels may rise within the first week in patients with type 2 diabetes, requiring alternative glucose-lowering medication and closer monitoring.
- Weight regain commonly occurs over 6–12 months following discontinuation, particularly without ongoing lifestyle modifications or alternative interventions.
- Discontinuation should be planned with a healthcare professional to ensure appropriate diabetes management strategies are in place and to monitor for complications.
Table of Contents
What Happens When You Stop Taking Ozempic
Ozempic (semaglutide) is a glucagon-like peptide-1 (GLP-1) receptor agonist licensed in the UK for the treatment of type 2 diabetes mellitus. It works by mimicking the action of the naturally occurring hormone GLP-1, which stimulates insulin secretion in response to food intake, suppresses glucagon release, slows gastric emptying, and reduces appetite through central nervous system pathways. When treatment with Ozempic is discontinued, these pharmacological effects gradually diminish as the medication is cleared from the body.
The half-life of semaglutide is approximately one week, meaning it takes around five weeks for the drug to be substantially eliminated from the system. During this period, the physiological changes induced by the medication begin to reverse. Patients may notice a return of previous symptoms related to their diabetes, including increased blood glucose levels, as the medication's glucose-lowering effects wane. The appetite-suppressing effects also diminish, which can lead to increased hunger and changes in eating patterns.
It is important to understand that stopping Ozempic does not cause the body to experience a 'rebound' effect in the traditional sense, but rather a gradual return to the metabolic state that existed before treatment commenced. For individuals who have been using Ozempic as part of their diabetes management plan, discontinuation should ideally be discussed with their GP or diabetes specialist to ensure appropriate alternative treatments are in place. Abrupt cessation without medical guidance may result in suboptimal glycaemic control and potential complications related to unmanaged type 2 diabetes.
Common Physical Effects After Stopping Ozempic
When Ozempic is discontinued, patients may experience several physical changes as the body adjusts to the absence of the medication. These effects are generally related to the reversal of the drug's pharmacological actions rather than true withdrawal symptoms. Understanding these changes can help patients prepare for the transition and recognise what is normal during this period.
Gastrointestinal changes are among the most commonly reported effects. Whilst taking Ozempic, many patients experience delayed gastric emptying, which contributes to feelings of fullness and satiety. After stopping the medication, gastric motility typically returns to baseline, which may initially feel unusual. Some individuals may notice changes in bowel habits as the digestive system readjusts, though evidence for specific post-discontinuation gastrointestinal effects is limited.
Blood glucose fluctuations represent another significant physical effect, particularly for those using Ozempic for diabetes management. As the medication's effects on glucose-dependent insulin secretion and glucagon suppression diminish, blood sugar levels may rise. Patients should monitor their glucose levels more frequently during the first few weeks after discontinuation, especially if no alternative diabetes medication has been prescribed. Symptoms of hyperglycaemia—including increased thirst, frequent urination, fatigue, and blurred vision—should prompt immediate contact with a healthcare professional. If glucose levels are persistently above 15 mmol/L, if ketones are present, or if you experience vomiting or drowsiness, seek same-day urgent care via NHS 111 or attend A&E if severely unwell.
Some patients report experiencing fatigue or changes in energy levels after stopping Ozempic, though there is no official link established between semaglutide discontinuation and specific energy-related symptoms. These experiences may relate more to dietary changes, alterations in blood glucose control, or psychological factors associated with stopping a medication that had been providing therapeutic benefit. If persistent fatigue occurs, it warrants medical review to exclude other causes such as anaemia, thyroid dysfunction, or inadequate diabetes control.
Weight Changes and Appetite After Discontinuation
Weight regain is one of the most frequently observed effects following Ozempic discontinuation, particularly among patients who experienced significant weight loss whilst on the medication. Clinical studies have demonstrated that semaglutide's weight-reducing effects are largely dependent on continued treatment, and cessation typically results in gradual weight increase. This occurs primarily because the appetite-suppressing mechanisms facilitated by GLP-1 receptor activation are no longer present.
The return of appetite after stopping Ozempic can be quite pronounced. Some patients report feeling considerably hungrier than they did whilst taking the medication. This is not a true rebound effect but rather reflects the removal of the central appetite suppression that semaglutide provides through its action on hypothalamic appetite centres. Additionally, the slowing of gastric emptying that contributed to prolonged satiety is reversed, meaning meals may feel less filling and hunger returns more quickly after eating.
The rate and extent of weight regain vary considerably between individuals and depend on multiple factors, including dietary habits, physical activity levels, metabolic rate, and whether alternative weight management strategies are implemented. Research from clinical trials suggests that without ongoing lifestyle modifications or alternative pharmacological interventions, many patients regain a substantial portion of the weight lost during treatment within 6–12 months of discontinuation, though it should be noted that much of this evidence comes from studies using the higher 2.4mg dose (Wegovy) licensed for weight management rather than the diabetes doses of Ozempic.
For patients concerned about weight regain, proactive strategies are essential. These include working with a dietitian to establish sustainable eating patterns, maintaining regular physical activity, addressing emotional or stress-related eating behaviours, and considering whether alternative weight management approaches might be appropriate. NICE guidance emphasises that pharmacological treatments for obesity should be used alongside comprehensive lifestyle interventions, and this principle remains important during the transition off medication. Patients should discuss their concerns about weight management with their GP, who can provide referrals to appropriate services such as NHS weight management programmes or dietetic support.
How Long Do Effects Last After Stopping Ozempic
The duration of effects following Ozempic discontinuation is closely related to the medication's pharmacokinetic properties. Semaglutide has an elimination half-life of approximately seven days, which means that after stopping treatment, it takes roughly five half-lives—or about five weeks—for the drug to be almost completely cleared from the body. However, the timeline for experiencing changes varies depending on which specific effect is being considered.
Appetite changes typically become noticeable within one to two weeks of the last dose, as semaglutide levels decline and the central appetite-suppressing effects diminish. Many patients may report increased hunger sensations during the second and third weeks after discontinuation. Gastrointestinal effects, such as the sensation of prolonged fullness after meals, similarly begin to fade within this timeframe as gastric emptying returns to normal rates.
Blood glucose control may deteriorate more rapidly, particularly in patients with type 2 diabetes who are not transitioned to alternative glucose-lowering medications. Some individuals notice rising blood sugar levels within the first week after stopping Ozempic, though the full impact on glycaemic control becomes more apparent over 2–4 weeks. This underscores the importance of having an alternative diabetes management plan in place before discontinuing the medication.
Weight regain typically follows a more gradual trajectory. Whilst some patients notice changes on the scales within the first month, more substantial weight regain usually occurs over several months. Studies examining weight trajectories after GLP-1 receptor agonist discontinuation suggest that most weight regain happens within the first 6–12 months, though individual patterns vary considerably.
It is worth noting that some metabolic improvements achieved during Ozempic treatment may persist longer than the medication itself remains in the system, particularly if patients have made sustainable lifestyle changes. Improvements in cardiovascular risk factors, for instance, may be maintained if healthy eating patterns and regular physical activity continue. However, without ongoing intervention—whether pharmacological or behavioural—most patients will gradually return towards their pre-treatment metabolic state.
Managing the Transition Off Ozempic Safely
Discontinuing Ozempic should ideally be a planned process undertaken in consultation with a healthcare professional rather than an abrupt cessation. For patients using Ozempic for type 2 diabetes management, it is essential to have an alternative treatment strategy in place to maintain glycaemic control. Your GP or diabetes specialist can advise on suitable alternatives, which might include other classes of glucose-lowering medications such as SGLT2 inhibitors, DPP-4 inhibitors, or adjustments to existing metformin therapy, depending on individual circumstances and HbA1c targets.
Regular blood glucose monitoring becomes particularly important during the transition period. Patients should check their blood sugar levels more frequently than usual—ideally before meals and two hours post-prandially—to identify any deterioration in control early. If blood glucose readings consistently exceed target ranges (typically 4–7 mmol/L fasting and less than 8.5 mmol/L two hours after meals, though individual targets vary), contact your healthcare team promptly for medication adjustment.
Patients taking insulin or sulfonylureas alongside Ozempic should be particularly cautious during the transition, as these medications may need dose adjustments to prevent either poor glucose control or hypoglycaemia. Such adjustments should always be made under clinical supervision.
Implementing or reinforcing lifestyle modifications can help mitigate some of the effects of stopping Ozempic, particularly regarding weight management. Consider working with a registered dietitian to develop a sustainable eating plan that addresses increased appetite without resorting to overly restrictive approaches, which often prove counterproductive. Focus on incorporating high-fibre foods, adequate protein at each meal, and plenty of vegetables to promote natural satiety. Regular physical activity—aiming for at least 150 minutes of moderate-intensity exercise weekly, as per UK Chief Medical Officers' guidelines—supports both weight maintenance and glycaemic control.
Psychological support may be valuable for some patients, particularly those who experience distress related to weight regain or feel they have 'failed' if discontinuation becomes necessary. Remember that stopping a medication does not represent personal failure; circumstances change, and treatment plans should adapt accordingly. If you experience low mood, anxiety about weight changes, or difficulty adjusting to the transition, speak with your GP about accessing appropriate support services.
Important considerations for specific situations: If you are planning pregnancy, semaglutide should be discontinued at least 2 months before attempting conception, as advised in the product information. Discuss with your healthcare provider if you are pregnant or planning pregnancy.
When to seek urgent medical advice: Contact your GP or diabetes team urgently if you experience symptoms of significantly elevated blood glucose (excessive thirst, frequent urination, unexplained weight loss, blurred vision, or fruity-smelling breath). If you have persistently high glucose levels (above 15 mmol/L), positive ketones, vomiting, or drowsiness, seek same-day urgent care via NHS 111 or attend A&E if severely unwell. Similarly, if you develop unexpected symptoms such as severe abdominal pain, persistent nausea or vomiting, or any other concerning changes after stopping Ozempic, seek medical review.
If you experience any suspected side effects after stopping Ozempic, you can report these through the MHRA Yellow Card Scheme (yellowcard.mhra.gov.uk or via the Yellow Card app).
Frequently Asked Questions
How quickly do you feel effects after stopping Ozempic?
Most patients notice increased appetite within one to two weeks of stopping Ozempic, whilst blood glucose changes may occur within the first week. The medication takes approximately five weeks to be substantially eliminated from the body.
Will I regain weight after stopping Ozempic?
Weight regain is common after stopping Ozempic, typically occurring gradually over 6–12 months. The extent of regain varies depending on lifestyle modifications, dietary habits, physical activity levels, and whether alternative weight management strategies are implemented.
Do I need to taper off Ozempic or can I stop suddenly?
Ozempic does not require tapering and can be stopped without dose reduction. However, discontinuation should be planned with your GP or diabetes specialist to ensure alternative diabetes management is in place and to monitor blood glucose levels during the transition.
The health-related content published on this site is based on credible scientific sources and is periodically reviewed to ensure accuracy and relevance. Although we aim to reflect the most current medical knowledge, the material is meant for general education and awareness only.
The information on this site is not a substitute for professional medical advice. For any health concerns, please speak with a qualified medical professional. By using this information, you acknowledge responsibility for any decisions made and understand we are not liable for any consequences that may result.
Heading 1
Heading 2
Heading 3
Heading 4
Heading 5
Heading 6
Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut aliquip ex ea commodo consequat. Duis aute irure dolor in reprehenderit in voluptate velit esse cillum dolore eu fugiat nulla pariatur.
Block quote
Ordered list
- Item 1
- Item 2
- Item 3
Unordered list
- Item A
- Item B
- Item C
Bold text
Emphasis
Superscript
Subscript












