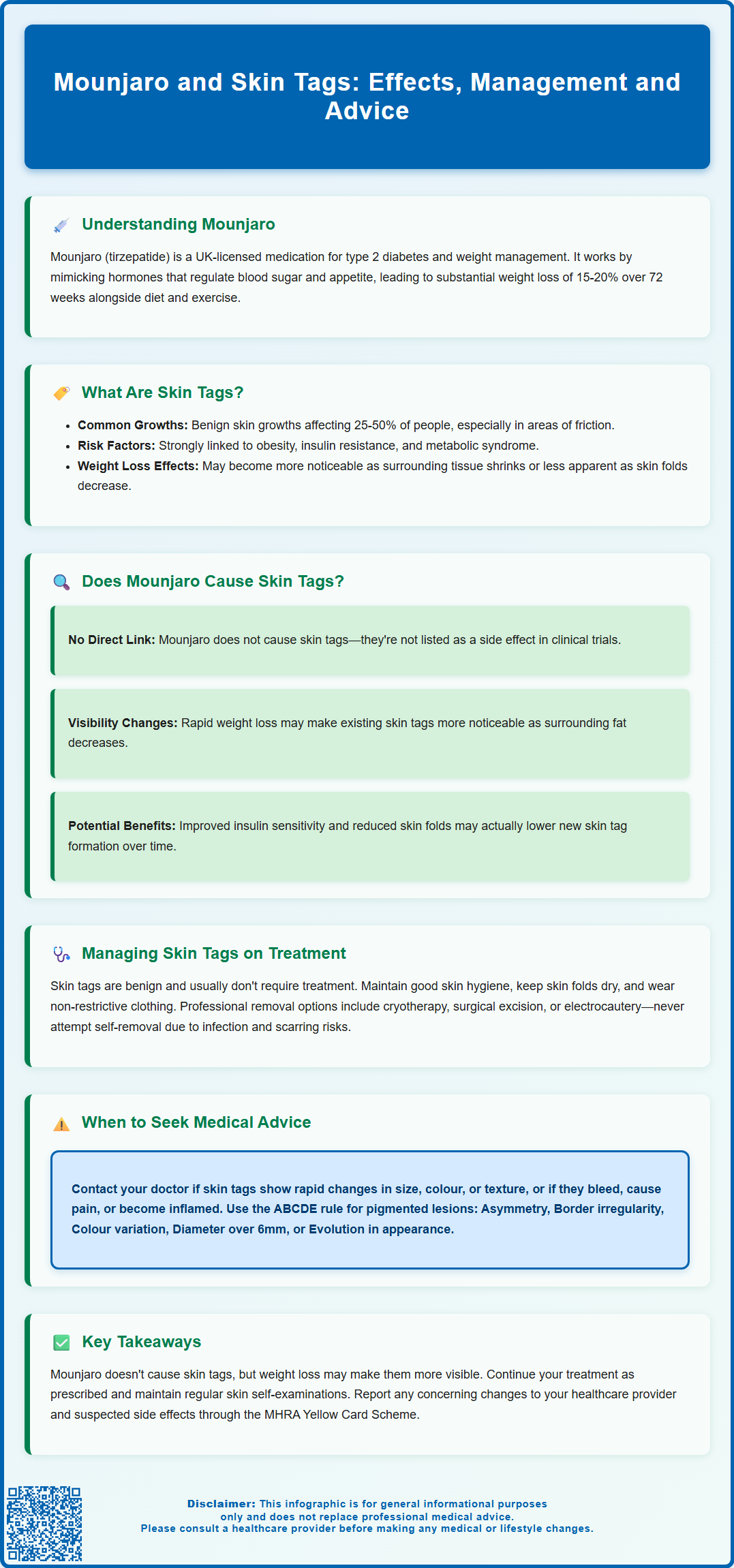
Mounjaro (tirzepatide) is a dual GIP/GLP-1 receptor agonist licensed in the UK for type 2 diabetes and weight management in adults with obesity or overweight with comorbidities. As patients experience substantial weight reduction, many notice various skin changes, including concerns about skin tags (acrochordons). Whilst Mounjaro primarily targets metabolic pathways to improve glycaemic control and reduce appetite, the significant physiological changes it produces can affect skin appearance. Understanding the relationship between Mounjaro and skin tags requires consideration of both the medication's direct effects and the indirect consequences of weight loss on dermatological health.
Summary: There is no established causal relationship between Mounjaro (tirzepatide) and skin tag development, though weight loss may alter their appearance or reduce new formation.
- Tirzepatide is a dual GIP/GLP-1 receptor agonist licensed for type 2 diabetes and weight management in the UK.
- Skin tags are benign growths strongly associated with obesity, insulin resistance, and metabolic syndrome.
- Weight loss from Mounjaro may make existing skin tags more noticeable or reduce mechanical friction that promotes new formation.
- Improved insulin sensitivity and reduced inflammation with treatment could theoretically decrease skin tag development over time.
- NHS removal of skin tags is typically not funded unless causing significant symptoms; private services are available for cosmetic concerns.
- Patients should seek medical advice for rapid changes, bleeding, pain, or numerous new growths appearing suddenly.
Table of Contents
Understanding Mounjaro (Tirzepatide) and Its Effects on the Skin
Mounjaro (tirzepatide) is a prescription medication licensed in the UK for the treatment of type 2 diabetes mellitus and, more recently, for weight management in adults with obesity (BMI ≥30 kg/m²) or overweight (BMI ≥27 kg/m²) with at least one weight-related comorbidity. It is prescribed as an adjunct to a reduced-calorie diet and increased physical activity. As a dual glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptor agonist, tirzepatide works by mimicking naturally occurring incretin hormones that regulate blood glucose levels and appetite.
The mechanism of action involves stimulating insulin secretion when blood glucose is elevated, suppressing glucagon release, slowing gastric emptying, and reducing appetite through central nervous system pathways. These combined effects lead to improved glycaemic control and significant weight reduction in many patients. Clinical trials have demonstrated that individuals using Mounjaro can experience substantial weight loss—with the SURMOUNT-1 trial showing approximately 15–20% of body weight loss over 72 weeks in adults without diabetes at the highest doses. Weight loss tends to be lower in people with type 2 diabetes, as shown in the SURMOUNT-2 trial. Tirzepatide represents a significant advance in pharmacological options for weight management.
As with any medication that produces rapid metabolic and physical changes, patients may notice various effects throughout their body, including changes to their skin. Skin-related concerns have emerged as a topic of interest among those prescribed Mounjaro, particularly regarding the development, persistence, or resolution of skin tags (acrochordons). Understanding how weight loss medications interact with dermatological health requires consideration of both the direct pharmacological effects of the drug and the indirect consequences of significant weight reduction. Patients should be aware that whilst Mounjaro primarily targets metabolic pathways, the substantial physiological changes it produces can manifest in various ways, including alterations to skin appearance and texture.
The Link Between Weight Loss Medications and Skin Tags
Skin tags (acrochordons) are small, benign growths of skin that typically appear in areas where skin rubs against skin or clothing, such as the neck, armpits, groin, eyelids, and under the breasts. These soft, flesh-coloured or slightly darker protrusions are extremely common, affecting approximately 25–50% of the general population, with prevalence increasing with age and body weight.
The relationship between obesity and skin tags is well established in dermatological literature. Insulin resistance, metabolic syndrome, and elevated body mass index (BMI) are all associated with increased skin tag formation. The proposed mechanisms include chronic low-grade inflammation, elevated insulin and insulin-like growth factor levels, and mechanical friction in skin folds—all of which are more prevalent in individuals with excess weight. Research has demonstrated that people with obesity are significantly more likely to develop multiple skin tags compared to those with healthy weight ranges.
When considering weight loss medications like Mounjaro, the relationship becomes more complex. As patients lose substantial amounts of weight, several dermatological changes may occur. Existing skin tags may become more noticeable as surrounding tissue reduces, or they may appear to diminish as skin folds decrease. Additionally, it is theoretically possible that the reduction in insulin resistance and inflammatory markers associated with weight loss could reduce the stimulus for new skin tag formation, though this has not been proven in clinical studies.
It is important to note that there is no official direct link established between GLP-1 or dual GIP/GLP-1 receptor agonists and the causation of skin tags. However, the dramatic physiological changes these medications produce—including alterations in hormone levels, skin elasticity, and body composition—mean that patients may observe various skin changes during treatment, which warrant appropriate clinical consideration and monitoring.
Can Mounjaro Cause or Reduce Skin Tags?
The question of whether Mounjaro directly causes or reduces skin tags requires careful examination of available evidence and biological plausibility. Currently, there is no established causal relationship between tirzepatide and the development of new skin tags. Skin tags are not listed among the common or uncommon adverse effects in the Mounjaro Summary of Product Characteristics approved by the MHRA or in clinical trial safety data.
However, patients may notice changes in existing skin tags or perceive new growths during Mounjaro treatment for several reasons. Rapid weight loss can alter skin tension and the appearance of pre-existing skin tags, making them more prominent or noticeable as surrounding adipose tissue diminishes. Conversely, as body weight decreases and skin folds reduce, the mechanical friction that contributes to skin tag formation may lessen, potentially reducing the development of new lesions over time.
From a metabolic perspective, Mounjaro's effects on insulin sensitivity and glucose metabolism could theoretically benefit skin health. As insulin resistance improves and inflammatory markers decrease with weight loss, the hormonal environment that promotes skin tag formation may become less favourable. Some patients anecdotally report that existing skin tags appear smaller or less prominent after sustained weight loss, though this observation lacks robust clinical trial evidence and should not be considered an expected outcome of treatment.
It is worth noting that the timeframe of treatment matters considerably. Patients who develop skin tags whilst taking Mounjaro may be experiencing a continuation of pre-existing metabolic conditions rather than a drug-induced effect. The natural history of skin tag development in individuals with obesity or type 2 diabetes means that new lesions may appear regardless of treatment. Any perceived increase in skin tags should be evaluated in the context of the patient's overall metabolic health, duration of obesity, family history, and other risk factors rather than automatically attributed to the medication itself.
Managing Skin Tags During Mounjaro Treatment
For patients who notice skin tags whilst taking Mounjaro, several management approaches are available, though it's important to emphasise that skin tags are benign and treatment is typically for cosmetic or comfort reasons rather than medical necessity.
Conservative management is often appropriate, particularly in the early stages of Mounjaro treatment when body composition is changing rapidly. Many patients find that as weight loss progresses and stabilises, the appearance of skin tags may improve naturally. Maintaining good skin hygiene, keeping skin folds dry, and wearing comfortable, non-restrictive clothing can help minimise irritation and reduce friction that might contribute to new skin tag formation.
If skin tags become bothersome, irritated, or cosmetically concerning, several removal options may be available:
-
Cryotherapy: Freezing the skin tag with liquid nitrogen
-
Surgical excision: Cutting off the skin tag under local anaesthetic
-
Electrocautery: Burning off the growth using heat
-
Ligation: Tying off the base to cut off blood supply
These procedures are generally quick and safe when performed by healthcare professionals. However, NHS removal of skin tags is typically not funded unless the lesions are causing significant symptoms such as bleeding, pain, or recurrent irritation, as they are considered a cosmetic concern. Availability varies by local Integrated Care Board (ICB) policies. Patients seeking removal for aesthetic reasons will usually need to access private dermatology services.
It's essential to have any skin growth properly diagnosed by a healthcare professional before considering treatment. People with diabetes should discuss any procedures with their clinician, as diabetes can increase infection risk and potentially delay wound healing.
Self-removal is strongly discouraged due to risks of infection, bleeding, and scarring. Over-the-counter skin tag removal products are available but should be used cautiously and only after a clinician has confirmed the lesion is indeed a skin tag rather than another type of growth. Patients should never attempt to cut, tie off, or otherwise remove skin tags themselves. Continuing Mounjaro treatment as prescribed remains important for metabolic health, and skin tag management should not interfere with the primary therapeutic goals of diabetes control or weight management.
When to Seek Medical Advice About Skin Changes on Mounjaro
Whilst skin tags are generally harmless, certain warning signs warrant prompt medical evaluation to exclude other dermatological conditions or complications. Patients taking Mounjaro should contact their GP or healthcare provider if they experience:
-
Rapid changes in the size, colour, or texture of existing skin growths
-
Bleeding, pain, or inflammation around skin tags without obvious trauma
-
Numerous new skin growths appearing suddenly over a short period
-
Asymmetrical or irregular lesions that don't resemble typical skin tags
-
Skin changes accompanied by systemic symptoms such as fevers, night sweats, unexplained fatigue, or other concerning features
For pigmented lesions, remember the ABCDE rule: seek advice if you notice Asymmetry, Border irregularity, Colour variation, Diameter >6mm, or Evolution/change in appearance.
It's important to remember that not all small skin growths are benign skin tags. Differential diagnoses include seborrhoeic keratoses, warts, moles (naevi), neurofibromas, and rarely, malignant lesions. Any uncertainty about the nature of a skin growth should prompt professional assessment. The NHS provides access to dermatology services through GP referral when clinical concern exists, and the two-week wait pathway is available for suspected skin cancers, in line with NICE guidance (NG12).
Patients should also discuss skin changes during routine diabetes or weight management reviews. Healthcare professionals monitoring Mounjaro treatment can assess whether skin changes are consistent with expected effects of weight loss or whether further investigation is needed. NICE guidance on diabetes management emphasises holistic patient care, which includes addressing dermatological concerns that may affect quality of life or treatment adherence.
Regular skin self-examination is advisable for all patients, particularly those with risk factors for skin conditions. Becoming familiar with your skin's normal appearance helps identify changes early. If you're uncertain whether a skin change requires medical attention, it's always better to seek advice rather than delay. Your GP or diabetes specialist nurse can provide reassurance, arrange appropriate examination, or refer to dermatology services if needed.
If you suspect you're experiencing a side effect from Mounjaro, report it to the MHRA through the Yellow Card Scheme (yellowcard.mhra.gov.uk or the Yellow Card app). Remember that managing your metabolic health with Mounjaro remains the priority, and most skin changes observed during treatment are benign and manageable with appropriate support.
Frequently Asked Questions
Does Mounjaro cause skin tags?
There is no established causal relationship between Mounjaro (tirzepatide) and skin tag development. Skin tags are not listed as an adverse effect in UK regulatory documentation or clinical trial data, though weight loss may alter the appearance of existing skin tags.
Can weight loss from Mounjaro reduce skin tags?
Weight loss may reduce mechanical friction in skin folds and improve insulin resistance, potentially decreasing new skin tag formation. Some patients report existing skin tags appear smaller after sustained weight loss, though this lacks robust clinical evidence.
Are skin tag removal procedures available on the NHS?
NHS removal of skin tags is typically not funded unless they cause significant symptoms such as bleeding, pain, or recurrent irritation, as they are considered cosmetic. Availability varies by local Integrated Care Board policies, and private dermatology services are usually required for aesthetic removal.
The health-related content published on this site is based on credible scientific sources and is periodically reviewed to ensure accuracy and relevance. Although we aim to reflect the most current medical knowledge, the material is meant for general education and awareness only.
The information on this site is not a substitute for professional medical advice. For any health concerns, please speak with a qualified medical professional. By using this information, you acknowledge responsibility for any decisions made and understand we are not liable for any consequences that may result.
Heading 1
Heading 2
Heading 3
Heading 4
Heading 5
Heading 6
Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut aliquip ex ea commodo consequat. Duis aute irure dolor in reprehenderit in voluptate velit esse cillum dolore eu fugiat nulla pariatur.
Block quote
Ordered list
- Item 1
- Item 2
- Item 3
Unordered list
- Item A
- Item B
- Item C
Bold text
Emphasis
Superscript
Subscript












