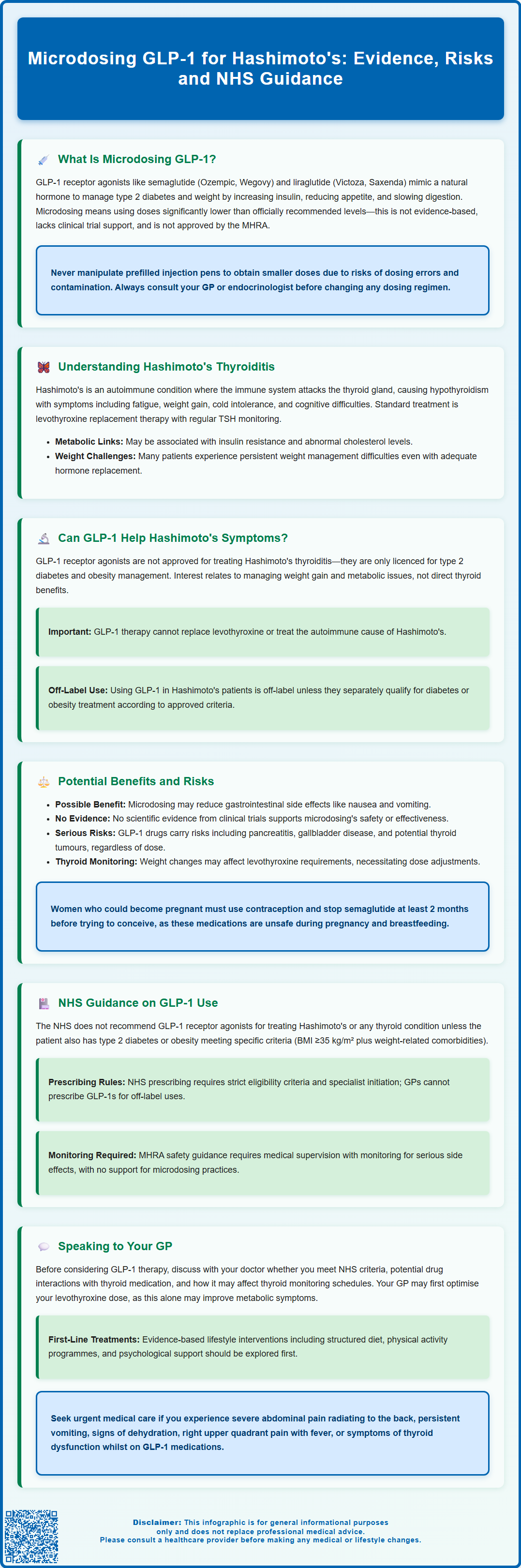
Microdosing GLP-1 for Hashimoto's has emerged as a topic of interest amongst patients seeking metabolic support for autoimmune thyroid disease. GLP-1 receptor agonists—medications approved for type 2 diabetes and obesity—are sometimes used at very low doses in the hope of reducing side effects whilst addressing weight gain and metabolic symptoms common in Hashimoto's thyroiditis. However, this practice lacks clinical evidence and regulatory approval. The NHS does not recommend GLP-1 medications for thyroid conditions unless concurrent diabetes or obesity criteria are met. This article examines the science, potential risks, and NHS guidance surrounding this off-label approach.
Summary: Microdosing GLP-1 for Hashimoto's is an unapproved, off-label practice lacking clinical evidence, and the NHS does not recommend GLP-1 receptor agonists for thyroid conditions without concurrent type 2 diabetes or obesity meeting specific criteria.
- GLP-1 receptor agonists (semaglutide, liraglutide, dulaglutide) are approved for type 2 diabetes and obesity, not Hashimoto's thyroiditis.
- Microdosing involves using doses below manufacturer-recommended levels without supporting clinical trial data on efficacy or safety.
- Hashimoto's thyroiditis is an autoimmune condition causing hypothyroidism, treated with levothyroxine replacement therapy according to NICE guidance.
- GLP-1 medications carry risks including pancreatitis, gallbladder disease, and potential thyroid C-cell tumours based on animal studies.
- Weight changes from GLP-1 therapy may require adjustment of levothyroxine doses, necessitating close thyroid function monitoring.
- NHS prescribing requires strict eligibility criteria; off-label use for Hashimoto's typically requires private prescription and specialist oversight.
Table of Contents
- What Is Microdosing GLP-1 and How Does It Work?
- Understanding Hashimoto's Thyroiditis and Metabolic Health
- Can GLP-1 Medications Help Manage Hashimoto's Symptoms?
- Potential Benefits and Risks of Microdosing GLP-1
- NHS Guidance on GLP-1 Use for Thyroid Conditions
- Speaking to Your GP About GLP-1 and Hashimoto's
- Frequently Asked Questions
What Is Microdosing GLP-1 and How Does It Work?
Glucagon-like peptide-1 (GLP-1) receptor agonists are a class of medications originally developed for type 2 diabetes management and, more recently, approved for weight management. These drugs mimic the action of naturally occurring GLP-1, a hormone released by the intestine in response to food intake. GLP-1 receptor agonists work through several mechanisms: they enhance insulin secretion when blood glucose levels are elevated, suppress glucagon release (which reduces glucose production by the liver), slow gastric emptying, and promote satiety through effects on appetite centres in the brain.
Commonly prescribed GLP-1 medications in the UK include semaglutide (Ozempic, Wegovy, and oral Rybelsus for type 2 diabetes), liraglutide (Victoza, Saxenda), and dulaglutide (Trulicity). These are typically administered via subcutaneous injection at standard therapeutic doses as recommended in the manufacturer's Summary of Product Characteristics (SmPC) and regulatory bodies such as the MHRA.
Microdosing refers to the practice of using significantly lower doses than those officially recommended—often a fraction of the standard starting dose. This differs from the approved low-dose titration schedules (such as starting semaglutide at 0.25mg) that manufacturers recommend to improve tolerability. Proponents suggest microdosing may reduce side effects whilst still providing metabolic benefits. However, it is crucial to understand that microdosing GLP-1 is not an evidence-based practice and lacks formal clinical trial data supporting its efficacy or safety. The MHRA has not approved any GLP-1 receptor agonist for use below the labelled doses, and this approach falls outside current prescribing guidelines. Importantly, patients should never attempt to manipulate prefilled injection pens to obtain smaller doses, as this risks dosing errors and contamination.
Patients considering any modification to standard dosing regimens should discuss this thoroughly with their GP or endocrinologist, as unsupervised dose adjustments may compromise treatment effectiveness and safety.
Understanding Hashimoto's Thyroiditis and Metabolic Health
Hashimoto's thyroiditis is the most common cause of hypothyroidism in the UK and represents an autoimmune condition where the immune system mistakenly attacks the thyroid gland. This progressive destruction of thyroid tissue leads to reduced production of thyroid hormones—primarily thyroxine (T4) and triiodothyronine (T3)—which are essential for regulating metabolism, energy production, and numerous bodily functions.
Patients with Hashimoto's typically present with symptoms of hypothyroidism including fatigue, weight gain, cold intolerance, constipation, dry skin, hair loss, and cognitive difficulties such as poor concentration or memory problems. Diagnosis is confirmed through blood tests showing elevated thyroid-stimulating hormone (TSH) and reduced free T4. The presence of thyroid peroxidase (TPO) antibodies or thyroglobulin antibodies supports an autoimmune aetiology but is not essential for diagnosis. According to NICE guidance (NG145), the standard treatment is levothyroxine replacement therapy, with dose titration based on regular TSH monitoring, typically checking TSH 6-8 weeks after dose changes with the aim of achieving a TSH within the reference range.
The metabolic complications of Hashimoto's extend beyond thyroid hormone deficiency. Research suggests that autoimmune thyroid disease may be associated with insulin resistance, dyslipidaemia, and cardiovascular risk factors. Many patients struggle with weight management even when thyroid hormone levels are adequately replaced, suggesting that thyroid dysfunction may trigger persistent metabolic changes. There appears to be a relationship between autoimmune thyroid disease and metabolic syndrome, with inflammation potentially playing a role, though more research is needed to fully understand these connections.
Patients should seek medical advice if they experience symptoms of thyroid dysfunction despite treatment, or if they develop concerning symptoms such as difficulty swallowing, voice changes, or a rapidly enlarging neck mass. Pregnant women with Hashimoto's typically require increased levothyroxine doses and should receive specialist endocrinology input.
Can GLP-1 Medications Help Manage Hashimoto's Symptoms?
There is currently no official link or approved indication for using GLP-1 receptor agonists specifically to treat Hashimoto's thyroiditis or its symptoms. The MHRA-approved indications for these medications are limited to type 2 diabetes mellitus and, for certain formulations, obesity management in adults with specific BMI thresholds as detailed in their SmPCs.
Some emerging research has explored potential connections between GLP-1 signalling and thyroid function. Preclinical studies have suggested that GLP-1 receptors are expressed in thyroid tissue, and animal models have indicated possible anti-inflammatory effects that could theoretically influence autoimmune processes. Nevertheless, these findings have not been replicated in robust human clinical trials, and the relevance to Hashimoto's management remains speculative.
The interest in GLP-1 medications for Hashimoto's patients often stems from their metabolic effects rather than direct thyroid benefits. Since many individuals with Hashimoto's experience weight gain and difficulty losing weight despite adequate thyroid hormone replacement, the appetite-suppressing properties of GLP-1 agonists may appear attractive. These medications may indirectly improve insulin sensitivity through weight loss and improved glycaemic control. Some patients report subjective improvements in energy levels and metabolic symptoms when using these medications, though such anecdotal reports cannot substitute for controlled clinical evidence.
It is essential to emphasise that any use of GLP-1 medications in Hashimoto's patients would be off-label unless the individual also meets criteria for type 2 diabetes or obesity treatment according to the medication's SmPC. Even then, NHS access may be further restricted by NICE technology appraisal guidance. Healthcare professionals must carefully weigh potential benefits against risks, ensure appropriate monitoring, and maintain realistic expectations about outcomes. Patients should not interpret GLP-1 therapy as a replacement for levothyroxine or as a treatment for the underlying autoimmune process.
Potential Benefits and Risks of Microdosing GLP-1
Advocates of microdosing GLP-1 suggest several potential advantages, primarily centred on reducing the gastrointestinal side effects commonly associated with standard doses. These adverse effects—including nausea, vomiting, diarrhoea, constipation, and abdominal discomfort—are dose-dependent and represent the most frequent reason for treatment discontinuation. By starting with very low doses and titrating slowly, some practitioners believe patients may achieve metabolic benefits whilst minimising tolerability issues.
Additionally, proponents argue that even sub-therapeutic doses might provide modest improvements in appetite regulation and weight management—outcomes that could benefit Hashimoto's patients struggling with metabolic complications. Some patients report feeling better able to adhere to dietary modifications and experience reduced cravings when using lower GLP-1 doses.
However, significant risks and limitations must be considered. Firstly, microdosing lacks evidence from randomised controlled trials, meaning efficacy and safety profiles are unknown. Using doses below the therapeutic threshold may provide insufficient metabolic benefit, essentially exposing patients to medication risks without meaningful clinical gain. Secondly, GLP-1 receptor agonists carry important safety considerations including risk of pancreatitis, gallbladder disease, and potential thyroid C-cell tumours (based on rodent studies, though human relevance remains uncertain).
For Hashimoto's patients specifically, there are additional concerns. Weight changes associated with GLP-1 treatment may necessitate adjustment of thyroid hormone replacement doses, as body weight influences levothyroxine requirements. Patients taking GLP-1 medications alongside insulin or sulfonylureas require careful monitoring for hypoglycaemia, and dose reductions of these medications may be necessary. Rapid improvement in glycaemic control may worsen diabetic retinopathy in some patients with type 2 diabetes. Dehydration from gastrointestinal side effects can increase the risk of acute kidney injury.
Importantly, GLP-1 medications are contraindicated in pregnancy and breastfeeding. Women of childbearing potential should use effective contraception, and semaglutide should be discontinued at least 2 months before a planned pregnancy. Patients should never attempt to manipulate prefilled pens to achieve microdoses, as this risks dosing errors and contamination. Unsupervised microdosing without appropriate medical oversight could lead to inadequate treatment. Patients must also consider the financial implications, as off-label use may not be NHS-funded, potentially incurring substantial private costs.
NHS Guidance on GLP-1 Use for Thyroid Conditions
The NHS does not currently recommend GLP-1 receptor agonists for the treatment of Hashimoto's thyroiditis or any thyroid condition in the absence of concurrent type 2 diabetes or obesity meeting specific criteria. NICE guidance provides clear recommendations for GLP-1 use, which focus exclusively on glycaemic control in type 2 diabetes (NG28) and weight management in obesity (TA875 for semaglutide, TA664 for liraglutide).
For type 2 diabetes, NICE recommends GLP-1 receptor agonists as second- or third-line therapy when metformin and other oral agents have not achieved adequate glycaemic control, or as an alternative to insulin in certain circumstances. For weight management, NICE technology appraisal guidance supports semaglutide 2.4 mg (Wegovy) for adults with at least one weight-related comorbidity and a BMI ≥35 kg/m² (or ≥30 kg/m² in exceptional circumstances), as part of a specialist weight management service. Treatment is typically limited to a maximum of 2 years. NICE TA664 provides similar guidance for liraglutide 3 mg (Saxenda) in selected high-risk groups.
Crucially, NHS prescribing of GLP-1 medications is subject to strict eligibility criteria and typically requires specialist initiation or shared care arrangements. GPs cannot routinely prescribe these medications for off-label indications such as Hashimoto's management. Patients seeking GLP-1 therapy outside approved indications would generally need to pursue private prescription, which carries significant costs that may vary considerably.
The MHRA safety guidance emphasises that GLP-1 receptor agonists should only be used under medical supervision with appropriate monitoring. For patients with thyroid disease, particular attention should be paid to thyroid function monitoring, as weight changes may influence thyroid hormone requirements. Healthcare professionals are advised to maintain vigilance for adverse effects including pancreatitis, gallbladder complications, and changes in renal function. Patients should be advised to report suspected side effects via the MHRA Yellow Card Scheme (yellowcard.mhra.gov.uk). There is no official guidance supporting microdosing practices, and such approaches would fall outside regulatory recommendations.
Speaking to Your GP About GLP-1 and Hashimoto's
If you are considering GLP-1 therapy for Hashimoto's-related metabolic symptoms, an open and informed discussion with your GP or endocrinologist is essential. Prepare for your appointment by documenting your current symptoms, particularly those related to weight management, energy levels, and metabolic health. Bring records of recent thyroid function tests (TSH, free T4) and note your current levothyroxine dose and how well-controlled your thyroid condition is.
Key questions to discuss include:
-
Whether you meet NHS criteria for GLP-1 prescription (type 2 diabetes or obesity with complications)
-
The evidence—or lack thereof—for GLP-1 use specifically in Hashimoto's management
-
Potential interactions between GLP-1 medications and thyroid hormone replacement
-
How GLP-1 therapy might affect your thyroid function monitoring schedule
-
Alternative evidence-based approaches to managing metabolic symptoms in Hashimoto's
-
The costs and practicalities of private prescription if NHS funding is not available
-
Contraception requirements if you are of childbearing potential (GLP-1 medications should be avoided in pregnancy and breastfeeding)
Your GP may recommend optimising your current thyroid management first, ensuring your levothyroxine dose is appropriate and that you are not over- or under-replaced. They might suggest referral to an endocrinologist for comprehensive metabolic assessment, or to a specialist weight management service if obesity is a significant concern. Evidence-based lifestyle interventions—including structured dietary advice, physical activity programmes, and psychological support—should be explored as first-line approaches.
When to seek urgent medical advice: Contact your GP promptly if you experience severe, persistent abdominal pain radiating to the back (possible pancreatitis); right upper quadrant pain, fever or jaundice (possible gallbladder disease); persistent vomiting or signs of dehydration; signs of thyroid dysfunction (significant fatigue, palpitations, heat intolerance); or any concerning symptoms whilst taking GLP-1 medications. If you have diabetes and are taking insulin or sulfonylureas alongside GLP-1 therapy, be alert for hypoglycaemia symptoms and discuss dose adjustments with your healthcare team.
If you are already using GLP-1 therapy through private prescription, ensure your GP is aware so they can coordinate monitoring and adjust your thyroid medication as needed. Remember that managing Hashimoto's effectively requires a collaborative, evidence-based approach—and your healthcare team is best placed to guide safe, appropriate treatment decisions tailored to your individual circumstances.
Frequently Asked Questions
Can GLP-1 medications treat Hashimoto's thyroiditis?
No, GLP-1 receptor agonists are not approved for treating Hashimoto's thyroiditis and lack clinical evidence supporting their use for autoimmune thyroid disease. They are only licensed for type 2 diabetes and obesity management.
Is microdosing GLP-1 safe for people with Hashimoto's?
Microdosing GLP-1 lacks clinical trial evidence and regulatory approval, making its safety profile unknown. Patients with Hashimoto's require careful monitoring as weight changes may affect levothyroxine requirements, and GLP-1 medications carry risks including pancreatitis and gallbladder disease.
Will the NHS prescribe GLP-1 medications for Hashimoto's symptoms?
The NHS does not prescribe GLP-1 medications for Hashimoto's unless you also meet strict criteria for type 2 diabetes or obesity with complications as outlined in NICE guidance. Off-label use typically requires private prescription.
The health-related content published on this site is based on credible scientific sources and is periodically reviewed to ensure accuracy and relevance. Although we aim to reflect the most current medical knowledge, the material is meant for general education and awareness only.
The information on this site is not a substitute for professional medical advice. For any health concerns, please speak with a qualified medical professional. By using this information, you acknowledge responsibility for any decisions made and understand we are not liable for any consequences that may result.
Heading 1
Heading 2
Heading 3
Heading 4
Heading 5
Heading 6
Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut aliquip ex ea commodo consequat. Duis aute irure dolor in reprehenderit in voluptate velit esse cillum dolore eu fugiat nulla pariatur.
Block quote
Ordered list
- Item 1
- Item 2
- Item 3
Unordered list
- Item A
- Item B
- Item C
Bold text
Emphasis
Superscript
Subscript












