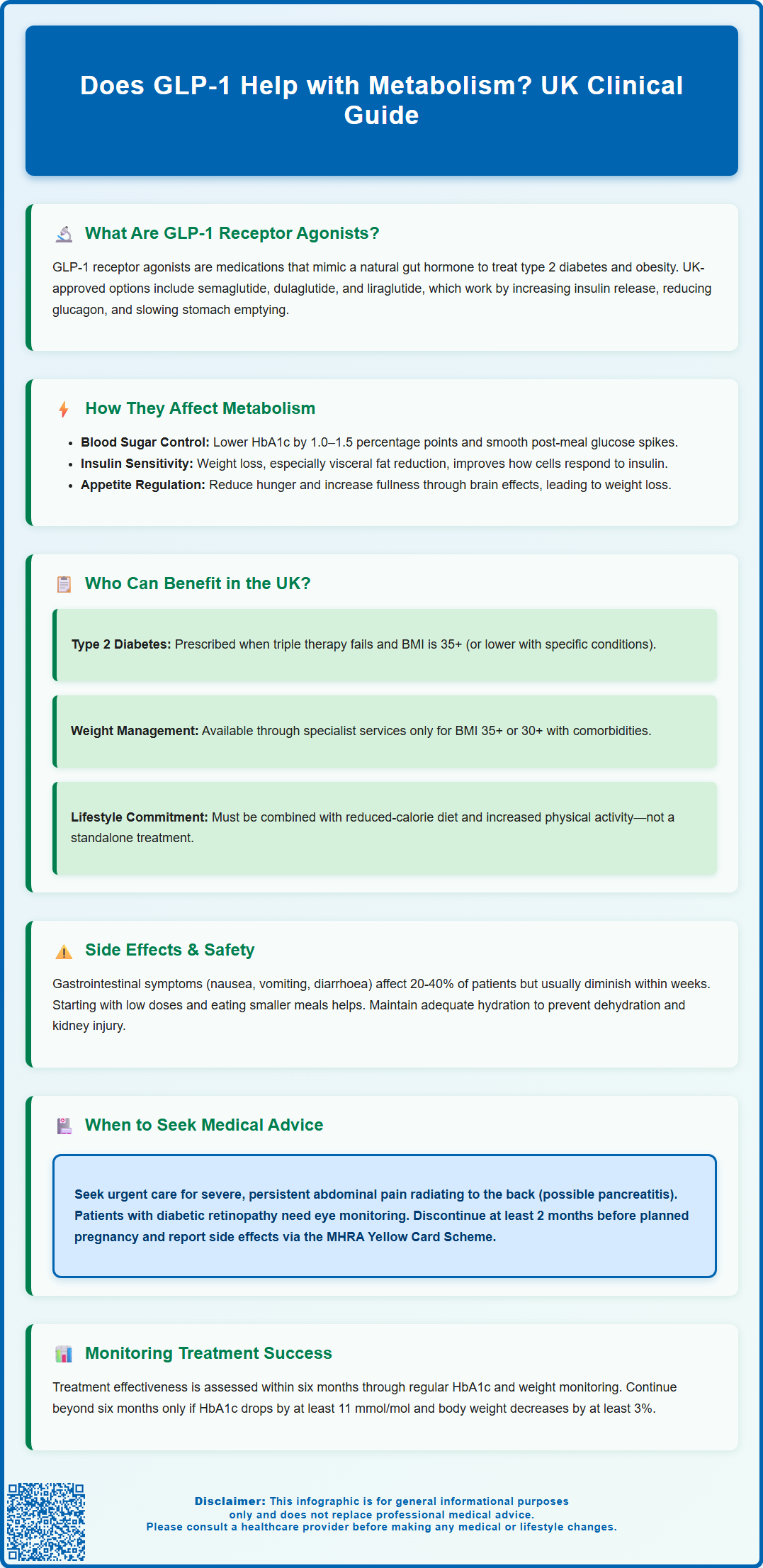
Glucagon-like peptide-1 (GLP-1) receptor agonists have emerged as important medications for managing type 2 diabetes and obesity in the UK. Many patients wonder: does GLP-1 help with metabolism? These medicines work by mimicking a natural hormone that influences multiple metabolic processes, including blood sugar regulation, insulin secretion, and appetite control. Licensed by the MHRA, GLP-1 receptor agonists such as semaglutide, dulaglutide, and liraglutide offer significant metabolic benefits when used appropriately. This article explores how GLP-1 medications affect metabolism, who may benefit from treatment, and important safety considerations for UK patients.
Summary: GLP-1 receptor agonists improve metabolism by enhancing glucose-dependent insulin secretion, suppressing glucagon release, delaying gastric emptying, and increasing satiety.
- GLP-1 receptor agonists are licensed in the UK for type 2 diabetes and weight management, including semaglutide, dulaglutide, and liraglutide
- These medications reduce HbA1c by approximately 1.0–1.5 percentage points and improve both fasting and post-meal glucose levels
- Weight loss associated with GLP-1 therapy can lead to secondary improvements in insulin resistance, particularly through reduction of visceral fat
- Common side effects include gastrointestinal symptoms such as nausea, vomiting, and diarrhoea, which typically diminish over several weeks
- NICE recommends continuation only if patients achieve at least 1.0 percentage point HbA1c reduction within six months of treatment initiation
- Patients with diabetic retinopathy require close monitoring, and GLP-1 medications should be discontinued at least two months before planned pregnancy
Table of Contents
What Are GLP-1 Receptor Agonists and How Do They Work?
Glucagon-like peptide-1 (GLP-1) receptor agonists are a class of medications that mimic the action of a naturally occurring hormone called GLP-1, which is produced in the intestines in response to food intake. These medicines have become increasingly important in the management of type 2 diabetes and, more recently, obesity. In the UK, several GLP-1 receptor agonists are licensed by the Medicines and Healthcare products Regulatory Agency (MHRA), each with specific indications: semaglutide (Ozempic for type 2 diabetes, Wegovy for weight management), dulaglutide (Trulicity for type 2 diabetes), and liraglutide (Victoza for type 2 diabetes, Saxenda for weight management).
The mechanism of action of GLP-1 receptor agonists is multifaceted and directly influences metabolic processes. These medications are resistant to breakdown by the enzyme DPP-4, allowing them to remain active in the body longer than natural GLP-1. When administered, they bind to GLP-1 receptors found throughout the body, particularly in the pancreas, brain, and gastrointestinal tract. This binding triggers several physiological responses that collectively improve metabolic function. Key metabolic effects include:
-
Enhanced insulin secretion from pancreatic beta cells in a glucose-dependent manner
-
Suppression of glucagon release from pancreatic alpha cells, reducing hepatic glucose production
-
Delayed gastric emptying, which slows the rate at which nutrients enter the bloodstream
-
Increased satiety through central nervous system pathways, reducing appetite and food intake
These combined actions help regulate blood glucose levels more effectively whilst also promoting weight loss in many patients. The glucose-dependent nature of insulin secretion means that GLP-1 receptor agonists carry a lower risk of hypoglycaemia compared to some other diabetes medications, as they primarily work when blood sugar levels are elevated.
It is important to note that GLP-1 receptor agonists are not indicated for type 1 diabetes or diabetic ketoacidosis.
Effects of GLP-1 on Blood Sugar and Insulin Sensitivity
GLP-1 receptor agonists exert significant beneficial effects on glucose metabolism and insulin sensitivity, which are central to their therapeutic value. Regarding blood sugar control, these medications work through multiple complementary pathways. The glucose-dependent stimulation of insulin secretion means that when blood glucose rises after meals, GLP-1 receptor agonists enhance the pancreatic response, facilitating more efficient glucose uptake by tissues. Simultaneously, the suppression of glucagon—a hormone that normally stimulates the liver to release stored glucose—helps prevent excessive glucose production between meals and overnight.
Clinical trials and NICE evidence reviews have demonstrated that GLP-1 receptor agonists can reduce HbA1c (a measure of average blood glucose over three months) by approximately 1.0–1.5 percentage points in people with type 2 diabetes. This improvement in glycaemic control occurs alongside reductions in both fasting and post-prandial (after-meal) glucose levels. The delayed gastric emptying effect further contributes to smoother glucose profiles by preventing rapid spikes in blood sugar following food consumption.
Insulin sensitivity refers to how effectively the body's cells respond to insulin. Whilst GLP-1 receptor agonists primarily work by enhancing insulin secretion rather than directly improving insulin sensitivity at the cellular level, the associated weight loss that often occurs with these medications can lead to secondary improvements in insulin resistance. Adipose tissue, particularly visceral fat, contributes to insulin resistance through the release of inflammatory mediators and free fatty acids. As patients lose weight on GLP-1 therapy, reductions in fat mass—especially around internal organs—can enhance the body's responsiveness to insulin.
Some important clinical considerations include:
-
People with pre-existing diabetic retinopathy should be monitored closely, as rapid improvement in blood glucose control with semaglutide has been associated with temporary worsening of retinopathy complications
-
GLP-1 receptor agonists may cause a modest increase in heart rate
-
These medications should not be used in combination with DPP-4 inhibitors, as recommended by NICE
NICE guidance recommends regular monitoring of HbA1c and body weight to assess treatment efficacy, with continuation of therapy dependent upon achieving meaningful clinical benefits within the first six months of treatment.
Who Can Benefit from GLP-1 Medications in the UK?
In the UK, GLP-1 receptor agonists are primarily prescribed for two main indications: type 2 diabetes mellitus and weight management in adults with obesity or overweight with weight-related comorbidities. NICE provides specific guidance on eligibility criteria to ensure these medications are used appropriately and cost-effectively within the NHS.
For type 2 diabetes management, NICE guideline NG28 recommends considering GLP-1 receptor agonists when:
-
Triple therapy with metformin and two other oral medications is ineffective, not tolerated or contraindicated
-
The patient has a body mass index (BMI) of 35 kg/m² or higher (with lower thresholds for people from certain ethnic backgrounds), or lower BMI with specific clinical circumstances where weight loss would benefit other obesity-related comorbidities
-
Insulin would have significant occupational implications
GLP-1 therapy should only be continued beyond six months if there has been a beneficial metabolic response, defined as a reduction in HbA1c of at least 11 mmol/mol (1.0 percentage point) and weight loss of at least 3% of initial body weight.
For weight management, specific GLP-1 formulations have restricted NHS availability:
-
Semaglutide 2.4 mg weekly (Wegovy) may be prescribed within specialist weight management services for adults with a BMI of at least 35 kg/m² (or 30 kg/m² with weight-related comorbidities) and at least one weight-related condition. Treatment is typically limited to a maximum of two years.
-
Liraglutide 3.0 mg daily (Saxenda) has similar restrictions and is also prescribed through specialist services with specific eligibility criteria.
It is important to note that Ozempic (semaglutide) is not licensed for weight management in the UK and should not be prescribed off-label for this purpose.
These medications are intended as an adjunct to a reduced-calorie diet and increased physical activity, not as standalone treatments. Patients must demonstrate commitment to lifestyle modification for treatment to be initiated and continued.
Important precautions include:
-
GLP-1 receptor agonists should not be used in patients with type 1 diabetes or diabetic ketoacidosis
-
Caution is advised in patients with a personal or family history of medullary thyroid carcinoma or multiple endocrine neoplasia syndrome type 2
-
These medications are not recommended in severe gastrointestinal disease
-
They should not be used during pregnancy or breastfeeding, and semaglutide should be discontinued at least 2 months before a planned pregnancy
Your GP or specialist will assess your individual circumstances to determine suitability.
Potential Side Effects and Safety Considerations
Whilst GLP-1 receptor agonists are generally well-tolerated, patients should be aware of potential adverse effects and important safety considerations. The most common side effects are gastrointestinal and typically occur during treatment initiation or dose escalation. These include:
-
Nausea (affecting 20–40% of patients initially)
-
Vomiting and diarrhoea
-
Constipation
-
Abdominal discomfort or bloating
-
Reduced appetite
These symptoms are usually mild to moderate and tend to diminish over several weeks as the body adjusts to the medication. Starting with a low dose and gradually increasing it, as prescribed, helps minimise gastrointestinal side effects. Eating smaller, more frequent meals and avoiding high-fat foods may also provide relief.
More serious but less common adverse effects require medical attention. Acute pancreatitis has been reported in association with GLP-1 receptor agonists, though a definitive causal relationship remains under investigation. Patients should seek urgent medical care if they experience severe, persistent abdominal pain that may radiate to the back, particularly if accompanied by vomiting. There have also been reports of gallbladder disease, including cholelithiasis (gallstones) and cholecystitis, possibly related to rapid weight loss.
Additional safety considerations include:
-
Diabetic retinopathy complications: People with pre-existing diabetic retinopathy using semaglutide should have appropriate ophthalmological monitoring, as rapid improvement in blood glucose control may be associated with temporary worsening of retinopathy
-
Dehydration and acute kidney injury: Significant gastrointestinal side effects may lead to dehydration; maintaining adequate fluid intake is important, and renal function should be monitored if severe vomiting or diarrhoea occurs
-
Thyroid concerns: The MHRA advises caution regarding potential risks of thyroid tumours based on animal studies, though there is no confirmed evidence of increased thyroid cancer risk in humans at therapeutic doses. Patients should report any symptoms such as a lump in the neck, hoarseness, or difficulty swallowing
-
Hypersensitivity reactions: Rare cases of serious allergic reactions have been reported
Hypoglycaemia risk is generally low with GLP-1 monotherapy due to the glucose-dependent mechanism of action. However, when combined with insulin or sulfonylureas, the risk increases, and dose adjustments of these concurrent medications may be necessary.
Patient safety advice includes:
-
Report persistent vomiting or signs of dehydration promptly to your healthcare provider
-
Seek urgent medical care for severe abdominal pain, especially if radiating to the back
-
Be aware of symptoms of hypoglycaemia if taking other diabetes medications
-
Inform your doctor if you are planning pregnancy, as GLP-1 receptor agonists should be discontinued (at least 2 months before planned pregnancy for semaglutide)
-
Attend regular follow-up appointments to monitor treatment response and side effects
Suspected adverse reactions should be reported via the MHRA Yellow Card Scheme (yellowcard.mhra.gov.uk or via the Yellow Card app).
Frequently Asked Questions
How do GLP-1 receptor agonists improve metabolic function?
GLP-1 receptor agonists improve metabolism by enhancing insulin secretion when blood glucose is elevated, suppressing glucagon to reduce liver glucose production, slowing gastric emptying to prevent blood sugar spikes, and increasing satiety to reduce food intake. These combined effects help regulate blood glucose levels more effectively whilst promoting weight loss in many patients.
Who is eligible for GLP-1 medications on the NHS?
For type 2 diabetes, NICE recommends GLP-1 receptor agonists when triple oral therapy is ineffective and BMI is 35 kg/m² or higher (with lower thresholds for certain ethnic groups). For weight management, specialist services may prescribe specific formulations for adults with BMI of at least 35 kg/m² (or 30 kg/m² with weight-related comorbidities) and at least one weight-related condition.
What are the most common side effects of GLP-1 medications?
The most common side effects are gastrointestinal, including nausea (affecting 20–40% initially), vomiting, diarrhoea, constipation, and abdominal discomfort. These symptoms typically occur during treatment initiation or dose escalation and usually diminish over several weeks as the body adjusts to the medication.
The health-related content published on this site is based on credible scientific sources and is periodically reviewed to ensure accuracy and relevance. Although we aim to reflect the most current medical knowledge, the material is meant for general education and awareness only.
The information on this site is not a substitute for professional medical advice. For any health concerns, please speak with a qualified medical professional. By using this information, you acknowledge responsibility for any decisions made and understand we are not liable for any consequences that may result.
Heading 1
Heading 2
Heading 3
Heading 4
Heading 5
Heading 6
Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut aliquip ex ea commodo consequat. Duis aute irure dolor in reprehenderit in voluptate velit esse cillum dolore eu fugiat nulla pariatur.
Block quote
Ordered list
- Item 1
- Item 2
- Item 3
Unordered list
- Item A
- Item B
- Item C
Bold text
Emphasis
Superscript
Subscript












