Mounjaro®
Dual-agonist support that helps curb appetite, hunger, and cravings to drive substantial, sustained weight loss.
- ~22.5% average body weight loss
- Significant weight reduction
- Improves blood sugar levels
- Clinically proven weight loss

Many people taking Ozempic (semaglutide) for type 2 diabetes expect significant appetite reduction, yet some continue to experience hunger despite treatment. Whilst this GLP-1 receptor agonist works by mimicking natural satiety hormones and slowing gastric emptying, individual responses vary considerably. Persistent hunger on Ozempic may result from insufficient dosing time, hormonal factors, psychological eating patterns, or lifestyle influences. Understanding why appetite suppression differs between patients—and recognising when to seek medical review—can help optimise diabetes management and treatment outcomes. This article explores the mechanisms behind Ozempic's effects on hunger and practical strategies for addressing continued appetite.
Summary: Continued hunger on Ozempic occurs because individual responses to GLP-1 receptor agonists vary considerably, and appetite suppression may be influenced by dosage, timing, hormonal factors, psychological eating patterns, and lifestyle habits.
Ozempic (semaglutide) is a glucagon-like peptide-1 (GLP-1) receptor agonist licensed in the UK for the treatment of type 2 diabetes mellitus. It works by mimicking the action of naturally occurring GLP-1, a hormone released from the intestine in response to food intake. This mechanism has several physiological effects that contribute to glycaemic control, with appetite reduction and weight loss occurring as secondary effects.
The appetite-suppressing properties of Ozempic occur through multiple pathways. Semaglutide acts on GLP-1 receptors in the hypothalamus—the brain region responsible for regulating hunger and satiety signals. By activating these receptors, the medication can increase feelings of fullness and reduce appetite. Additionally, Ozempic slows gastric emptying, meaning food remains in the stomach for longer periods, which typically prolongs the sensation of satiety after meals, though this effect may diminish somewhat with ongoing treatment.
Clinical trials suggest that many patients may experience some reduction in appetite during treatment, with effects potentially becoming more noticeable as the dose is titrated upwards. However, individual responses vary considerably. The medication also enhances glucose-dependent insulin secretion and suppresses inappropriate glucagon release, contributing to improved blood glucose control. These metabolic effects work alongside any appetite reduction to support weight loss in many patients.
It is important to recognise that whilst Ozempic may reduce appetite in many users, it is not universally effective for appetite suppression. The degree of hunger reduction experienced can differ substantially between individuals, and some patients may notice minimal changes to their baseline appetite despite being on a therapeutic dose.
Several physiological and pharmacological factors may explain why some individuals continue to experience hunger whilst taking Ozempic. Understanding these reasons can help patients and clinicians identify potential solutions.
Individual variation in response to GLP-1 receptor agonists is a primary consideration. Differences in baseline metabolic function and other individual factors may influence how effectively semaglutide suppresses appetite in different people. Some patients may have compensatory mechanisms that maintain hunger signals despite GLP-1 receptor activation.
Insufficient time on treatment is another common explanation. Ozempic is initiated at a low dose (0.25 mg weekly for 4 weeks), then increased to 0.5 mg weekly as the standard maintenance dose. For patients requiring additional glycaemic control, the dose may be further increased to 1 mg or 2 mg weekly, with at least 4 weeks between dose adjustments. The appetite-suppressing effects may become more pronounced at higher doses, though dose increases should only be made under clinical supervision for glycaemic control, not specifically for appetite suppression.
Hormonal and metabolic factors can also interfere with appetite regulation. Conditions such as insulin resistance, polycystic ovary syndrome (PCOS), hypothyroidism, or untreated endocrine disorders may influence the appetite-suppressing effects of Ozempic. Additionally, certain medications—including some antidepressants, antipsychotics, and corticosteroids—can increase appetite and potentially counteract semaglutide's effects.
Psychological hunger versus physiological hunger represents an important distinction. Ozempic primarily addresses physiological hunger signals but may have limited impact on emotional eating, stress-related food cravings, or habitual eating patterns. Patients may interpret these psychological drives to eat as continued hunger, even when physiological satiety signals are present.
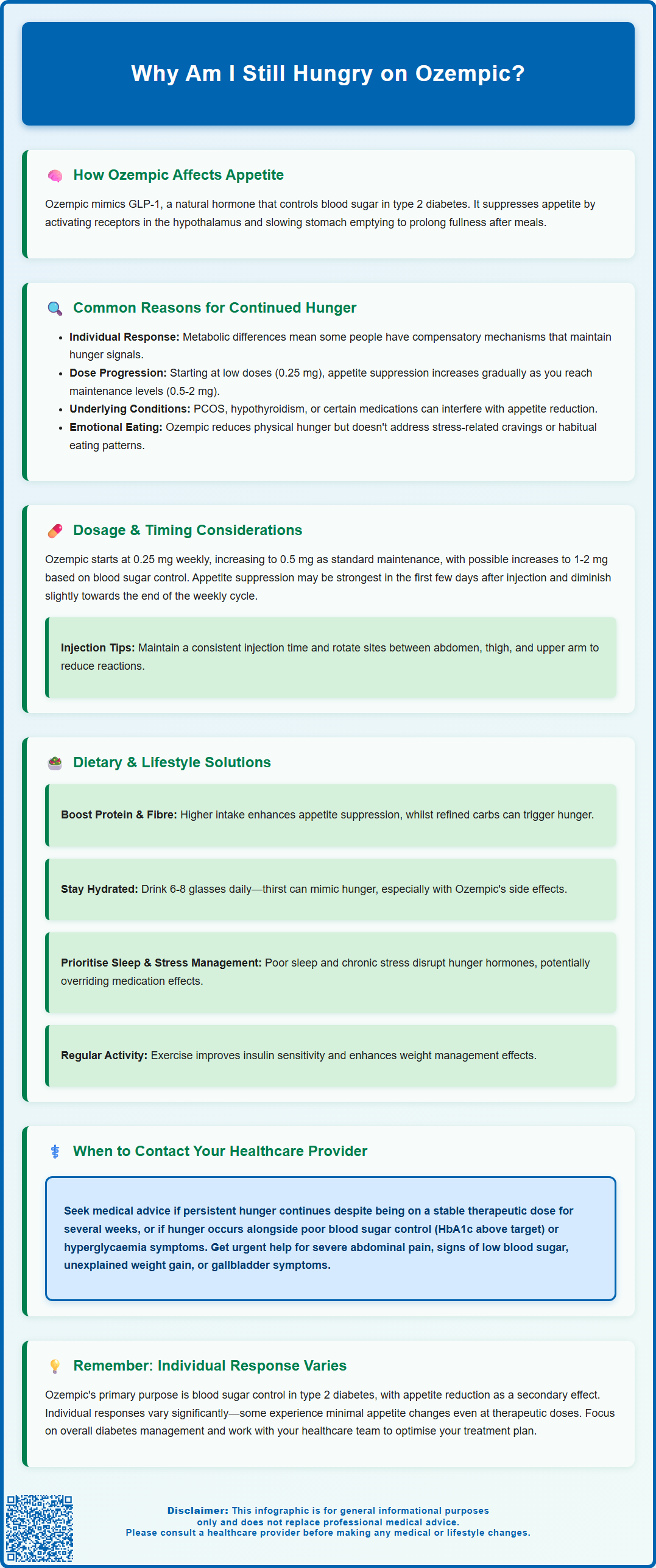
The relationship between Ozempic dosage and appetite suppression may follow a dose-dependent pattern, though individual responses vary considerably. The standard titration schedule begins with 0.25 mg once weekly for four weeks, primarily to improve gastrointestinal tolerability. This initial dose is generally sub-therapeutic for glycaemic control.
After the first month, the dose is typically increased to 0.5 mg weekly, which represents the standard maintenance dose for type 2 diabetes management. For patients requiring additional glycaemic control, the dose may be further increased to 1 mg weekly after at least four weeks on the 0.5 mg dose. In some cases, if additional glycaemic control is needed, the dose may be increased to 2 mg weekly after at least four weeks on the 1 mg dose. All dose adjustments should be made under clinical supervision based on glycaemic control needs, not for appetite or weight management purposes.
Timing of administration can influence how patients perceive hunger throughout the week. Ozempic has a half-life of approximately one week, providing relatively stable blood levels. However, some patients report variations in appetite suppression during the weekly dosing cycle, with effects potentially being strongest in the first few days post-injection and diminishing slightly towards the end of the week.
Individual pharmacokinetic differences may affect drug absorption and metabolism. The MHRA-approved prescribing information indicates that Ozempic can be administered at any time of day, with or without meals, though consistency in timing is recommended. If a dose is missed, it should be administered as soon as possible within 5 days after the missed dose. If more than 5 days have passed, the missed dose should be skipped, and the next dose administered on the regularly scheduled day.
It is worth noting that there is no official link between injection timing (morning versus evening) and appetite control, though some patients report subjective preferences. For optimal administration, injection sites (abdomen, thigh, or upper arm) should be rotated to reduce the risk of injection site reactions.
Even when taking Ozempic, dietary composition and lifestyle habits significantly influence hunger levels and the medication's effectiveness. Addressing these factors can enhance appetite control and optimise treatment outcomes.
Macronutrient balance plays a crucial role in satiety. Diets higher in protein and fibre tend to promote greater fullness and work synergistically with Ozempic's appetite-suppressing effects. Conversely, diets high in refined carbohydrates and sugars can cause rapid blood glucose fluctuations, potentially triggering hunger signals despite medication use. The NHS recommends that adults consume at least 30g of fibre daily and include protein sources at each meal to support satiety.
Meal timing and frequency also matter. Some patients find that eating smaller, more frequent meals helps manage hunger, whilst others benefit from a structured three-meal pattern. Individual meal patterns should be developed based on personal preference and effectiveness. Regular meal patterns help stabilise blood glucose levels and support the medication's mechanism of action.
Hydration status is frequently overlooked. Thirst can sometimes be misinterpreted as hunger, and adequate fluid intake (approximately 6–8 glasses daily as recommended by the NHS) is essential for optimal metabolic function. Additionally, gastrointestinal side effects of Ozempic such as nausea, vomiting or diarrhoea can lead to dehydration, particularly if accompanied by reduced food intake.
Sleep quality and stress management profoundly affect appetite regulation. Poor sleep increases ghrelin (the hunger hormone) and decreases leptin (the satiety hormone), potentially overriding Ozempic's appetite-suppressing effects. Chronic stress elevates cortisol, which can increase appetite and promote cravings for high-calorie foods. Addressing these lifestyle factors through good sleep hygiene and stress-reduction techniques may enhance the medication's effectiveness.
Physical activity levels influence both appetite and metabolic response to Ozempic. Regular exercise improves insulin sensitivity and can enhance the medication's effects on weight management, though intense exercise may temporarily increase appetite in some individuals.
Whilst some degree of continued hunger on Ozempic may be normal, particularly during dose titration, certain situations warrant discussion with your GP, practice nurse, or diabetes specialist nurse (DSN).
Persistent hunger despite adequate dosing should be reviewed. If you have been on a stable therapeutic dose (0.5 mg, 1 mg or 2 mg weekly) for several weeks and experience no reduction in appetite, your healthcare provider may need to reassess your treatment plan. This could involve checking for underlying conditions affecting appetite regulation, reviewing concurrent medications, or considering alternative therapeutic approaches.
Inadequate glycaemic control alongside continued hunger requires prompt attention. If your HbA1c levels remain above target despite treatment, or if you experience symptoms of hyperglycaemia (increased thirst, frequent urination, fatigue), contact your healthcare team. NICE guidelines recommend regular monitoring of glycaemic control in patients with type 2 diabetes, typically every 3–6 months.
Concerning symptoms that should trigger medical review include:
Severe or persistent abdominal pain, which could indicate pancreatitis (a rare but serious adverse effect)—seek urgent medical help via NHS 111, 999, or A&E as appropriate
Signs of hypoglycaemia (if taking Ozempic with insulin or sulphonylureas)
Unexplained weight gain or inability to lose weight despite dietary adherence
New or worsening visual symptoms, especially if you have pre-existing diabetic retinopathy
Right upper abdominal pain, fever or jaundice, which could indicate gallbladder problems
Medication review is important if you are taking other drugs that may affect appetite or interact with Ozempic. Your healthcare provider can assess whether dose adjustments or alternative medications might be appropriate.
Finally, if continued hunger is significantly affecting your quality of life, mental health, or ability to manage your diabetes effectively, do not hesitate to seek support. Your healthcare team can provide referrals to dietitians, diabetes specialist nurses, or psychological support services as needed. Effective diabetes management requires a personalised approach, and open communication with your healthcare providers is essential for optimising your treatment outcomes.
If you experience any suspected side effects from Ozempic, report them to the MHRA Yellow Card scheme, which helps monitor the safety of medicines in the UK.
Appetite-suppressing effects may become more noticeable as the dose is titrated upwards over several weeks, with at least four weeks required between dose adjustments from 0.25 mg to 0.5 mg, 1 mg, or 2 mg weekly. Individual responses vary considerably, and some patients may experience minimal appetite changes despite therapeutic dosing.
Dose adjustments should only be made under clinical supervision based on glycaemic control needs, not specifically for appetite suppression or weight management. Speak to your GP or diabetes specialist nurse if you have concerns about persistent hunger on your current dose.
Focus on dietary factors such as increasing protein and fibre intake, maintaining adequate hydration, improving sleep quality, and managing stress, as these significantly influence hunger levels. If appetite remains unchanged after several weeks on a therapeutic dose, consult your healthcare provider to review your treatment plan and check for underlying factors affecting appetite regulation.
The health-related content published on this site is based on credible scientific sources and is periodically reviewed to ensure accuracy and relevance. Although we aim to reflect the most current medical knowledge, the material is meant for general education and awareness only.
The information on this site is not a substitute for professional medical advice. For any health concerns, please speak with a qualified medical professional. By using this information, you acknowledge responsibility for any decisions made and understand we are not liable for any consequences that may result.
Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut aliquip ex ea commodo consequat. Duis aute irure dolor in reprehenderit in voluptate velit esse cillum dolore eu fugiat nulla pariatur.
Block quote
Ordered list
Unordered list
Bold text
Emphasis
Superscript
Subscript