Many individuals seeking weight management solutions wonder whether combining prescription medications with over-the-counter supplements is safe and effective. Saxenda (liraglutide) is a licensed GLP-1 receptor agonist prescribed for obesity management, whilst Lipozene is a glucomannan-based dietary supplement. There is no official UK guidance on using Saxenda and Lipozene together, and theoretical concerns exist regarding additive gastrointestinal effects and delayed gastric emptying. This article examines the pharmacological considerations, potential risks, and evidence-based approaches to weight management. Always consult your GP or prescribing clinician before combining prescription weight loss medications with any supplements to ensure your treatment plan remains safe and appropriate for your individual circumstances.
Summary: There is no official UK guidance on combining Saxenda and Lipozene, and concurrent use may increase gastrointestinal side effects due to their overlapping mechanisms affecting gastric emptying and satiety.
- Saxenda is a prescription GLP-1 receptor agonist licensed for weight management, whilst Lipozene is an over-the-counter glucomannan fibre supplement regulated as a food product.
- Both products slow gastric emptying and promote satiety, potentially causing additive gastrointestinal effects including severe bloating, nausea, constipation, or theoretical obstruction risk.
- The Saxenda Summary of Product Characteristics advises against concomitant use with other weight management medicines, a caution relevant to supplements.
- NICE recommends Saxenda only as an adjunct to lifestyle changes within specialist weight management services, with continuation dependent on achieving at least 5% weight loss after 12 weeks.
- Patients must consult their GP or prescribing clinician before combining Saxenda with any over-the-counter weight loss supplements to assess individual risks and medication interactions.
Table of Contents
What Are Saxenda and Lipozene?
Saxenda (liraglutide 3.0 mg) is a prescription-only medicine licensed in the UK for weight management in adults with obesity (BMI ≥30 kg/m²) or overweight (BMI ≥27 kg/m²) with weight-related comorbidities such as type 2 diabetes, hypertension, dyslipidaemia, obstructive sleep apnoea or prediabetes. It is also licensed for adolescents aged 12 to <18 years with body weight above 60 kg and obesity. Saxenda belongs to a class of medications called glucagon-like peptide-1 (GLP-1) receptor agonists. It works by mimicking the action of GLP-1, a naturally occurring hormone that regulates appetite and food intake. The medication slows gastric emptying, increases feelings of fullness, and reduces hunger signals in the brain, thereby helping patients consume fewer calories. It is administered as a once-daily subcutaneous injection and is intended to be used alongside a reduced-calorie diet and increased physical activity.
Lipozene, by contrast, is an over-the-counter dietary supplement regulated as a food supplement under UK Food Standards Agency (FSA) oversight, not as a medicine by the Medicines and Healthcare products Regulatory Agency (MHRA). Its primary ingredient is glucomannan, a water-soluble dietary fibre derived from the konjac root (Amorphophallus konjac). The European Food Safety Authority (EFSA) has approved a health claim that glucomannan can contribute to weight loss when 3g is consumed daily in three 1g doses, alongside a calorie-restricted diet, and taken with 1-2 glasses of water before meals. Glucomannan is thought to work by absorbing water in the digestive tract, forming a viscous gel that may promote feelings of fullness and reduce appetite. Unlike Saxenda, Lipozene has not undergone the rigorous clinical trials required for pharmaceutical approval, and its efficacy and safety profile are not established to the same standard as prescription medications.
The fundamental difference between these products lies in their regulatory status, evidence base, and mechanism of action. Saxenda is a clinically validated pharmaceutical intervention with established efficacy data from randomised controlled trials, whilst Lipozene is a food supplement with limited high-quality evidence supporting its weight loss claims. Understanding these distinctions is essential when considering their use, either individually or in combination.
Can You Take Saxenda and Lipozene Together?
There is no official guidance from the MHRA, NICE (National Institute for Health and Care Excellence), or the manufacturer of Saxenda regarding the concurrent use of liraglutide with glucomannan-containing supplements such as Lipozene. However, the Saxenda Summary of Product Characteristics (SmPC) does state that concomitant use with other weight management medicines is not recommended. While Lipozene is a supplement rather than a medicine, this caution is still relevant to consider.
From a pharmacological perspective, there are theoretical concerns about combining these products. Saxenda already slows gastric emptying as part of its mechanism of action, which delays the passage of food through the stomach and contributes to satiety. Glucomannan similarly affects gastrointestinal transit by forming a gel-like substance that increases the viscosity of stomach contents. When used together, these effects could potentially be additive, leading to excessive slowing of gastric emptying. This may result in uncomfortable gastrointestinal symptoms such as severe bloating, abdominal distension, nausea, or constipation.
Furthermore, glucomannan's gel-forming properties may theoretically interfere with the absorption of other medications or nutrients. Whilst Saxenda is injected subcutaneously and therefore not subject to gastrointestinal absorption issues, patients taking other oral medications alongside these products should be aware of potential interactions. The EFSA recommends taking glucomannan with 1-2 glasses of water before meals to prevent oesophageal obstruction, and this risk may be compounded when gastric emptying is already delayed. If you take critical oral medicines (such as levothyroxine, warfarin, or certain diabetes medications), it would be advisable to separate these by several hours from any glucomannan supplement.
Patients with swallowing difficulties or pre-existing gastrointestinal conditions such as gastroparesis should be particularly cautious, as GLP-1 receptor agonists like Saxenda are generally not recommended in severe gastrointestinal disease.
It is essential to consult your GP or prescribing clinician before combining Saxenda with any over-the-counter weight loss supplements, including Lipozene. Your healthcare provider can assess your individual circumstances, review your complete medication list, and provide personalised advice based on your medical history and weight management goals.
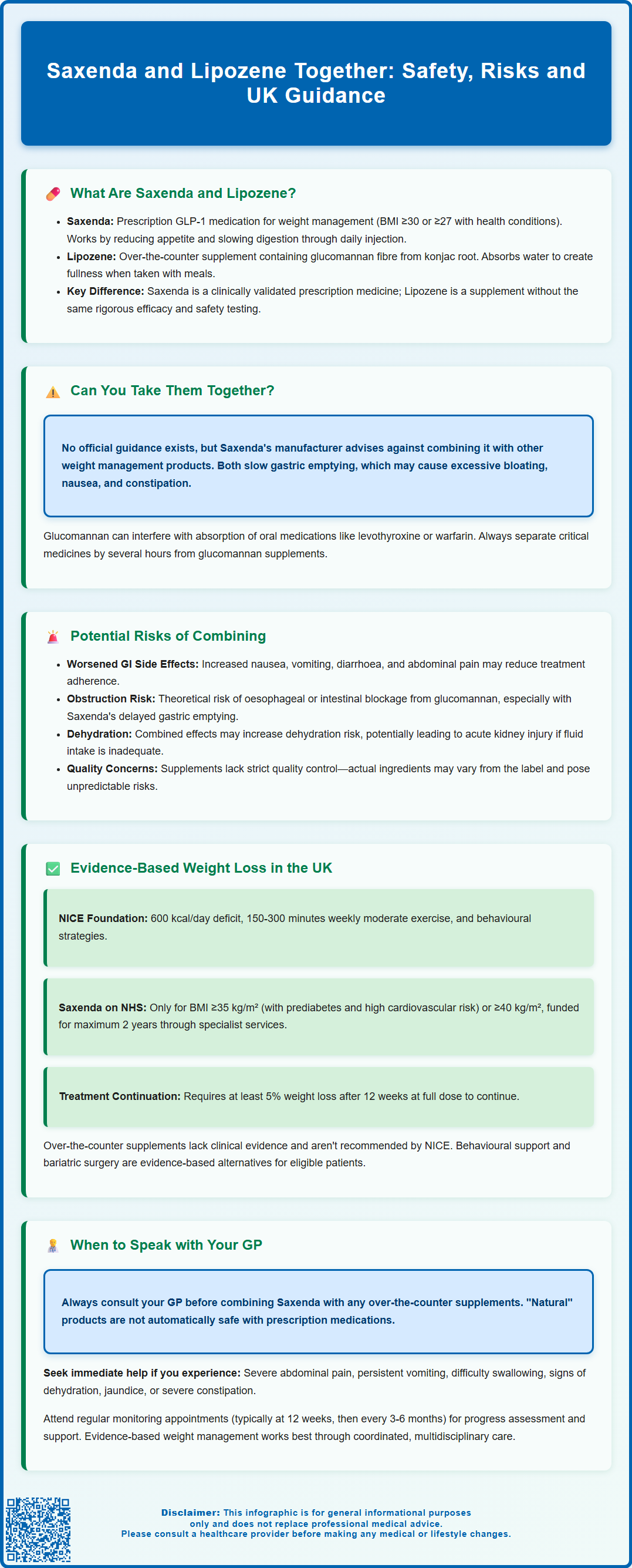
Potential Risks of Combining Saxenda and Lipozene
Combining Saxenda and Lipozene may increase the risk of gastrointestinal adverse effects. Saxenda commonly causes nausea (reported in approximately 40% of patients in clinical trials), vomiting, diarrhoea, constipation, and abdominal pain, particularly during dose escalation. These effects typically diminish over time as the body adjusts to the medication. Glucomannan can also cause gastrointestinal symptoms, including bloating, flatulence, and loose stools due to its high fibre content and water-absorbing properties. When both products are used simultaneously, patients may experience an intensification of these symptoms, potentially affecting treatment adherence and quality of life.
There is also a theoretical risk of oesophageal or intestinal obstruction with glucomannan supplements, particularly if taken without sufficient fluid. The EFSA has issued warnings about this risk, especially in individuals with swallowing difficulties or oesophageal disorders. Given that Saxenda delays gastric emptying, the prolonged presence of glucomannan in the upper gastrointestinal tract could potentially increase the risk of obstruction, though this remains a theoretical concern without documented case reports.
Saxenda carries additional risks not present with fibre supplements, including gallbladder disorders (cholelithiasis and cholecystitis), dehydration that may lead to acute kidney injury (particularly if experiencing gastrointestinal side effects), and potential hypoglycaemia when used alongside certain diabetes medications. The combination with a high-fibre supplement could potentially exacerbate dehydration risk if adequate fluid intake is not maintained.
Nutrient absorption may be affected when high-dose fibre supplements are used regularly. Glucomannan can bind to certain nutrients and medications in the gut, potentially reducing their bioavailability. Whilst this is less of a concern for injected medications like Saxenda, patients taking oral medications for comorbid conditions should be aware of potential interactions. Timing of medication administration becomes crucial in such scenarios, and separating glucomannan from critical oral medicines by several hours is advisable.
Additionally, the quality control of dietary supplements in the UK varies. While regulated as food supplements under the Food Standards Agency, they are not subject to the same stringent manufacturing standards as prescription medications. Variability in glucomannan content, presence of undeclared ingredients, or contamination could pose unforeseen risks, particularly when combined with prescription medications. Patients should be aware that the actual composition of Lipozene may differ from what is stated on the label, as supplements are not subject to the same batch-testing requirements as licensed medicines.
Evidence-Based Weight Loss Approaches in the UK
NICE guidelines (CG189) recommend a comprehensive, multicomponent approach to weight management that addresses dietary intake, physical activity, and behavioural change. For adults with obesity, initial interventions should focus on lifestyle modifications, including a reduced-calorie diet (typically a 600 kcal/day deficit), increased physical activity (aiming for 150–300 minutes of moderate-intensity activity per week per UK Chief Medical Officers' guidelines), and behavioural strategies to support long-term habit change. These interventions form the foundation of effective weight management and should be maintained even when pharmacological treatments are introduced.
Pharmacological interventions such as Saxenda are recommended by NICE only as an adjunct to lifestyle changes, not as standalone treatments. According to NICE Technology Appraisal 664, liraglutide 3.0 mg is recommended as an option for weight management within its marketing authorisation in adults with a BMI of at least 35 kg/m² and prediabetes plus high risk of cardiovascular disease, or a BMI of at least 40 kg/m² without diabetes. Treatment should be initiated and supervised within specialist weight management services (Tier 3), and continued only if patients lose at least 5% of their initial body weight after 12 weeks at the full 3.0 mg daily dose. NHS funding is available for a maximum treatment duration of 2 years. Other pharmacological options approved by NICE include orlistat, and more recently, semaglutide (Wegovy) for weight management.
Behavioural support programmes are integral to successful weight management. These may include individual or group-based interventions delivered by trained practitioners, focusing on goal-setting, self-monitoring, stimulus control, and relapse prevention strategies. The NHS provides various weight management services, including the NHS Digital Weight Management Programme, which offers evidence-based support for eligible patients.
Bariatric surgery represents another evidence-based option for appropriate patients, typically those with BMI ≥40 kg/m² or ≥35 kg/m² with significant comorbidities who have not achieved adequate weight loss through non-surgical interventions. Procedures such as gastric bypass or sleeve gastrectomy have robust evidence for sustained weight loss and improvement in obesity-related conditions.
Importantly, dietary supplements and over-the-counter weight loss products generally lack the evidence base to be recommended in clinical guidelines. Patients should be cautious about claims made by supplement manufacturers and prioritise interventions with established efficacy and safety profiles.
When to Speak with Your GP About Weight Management
You should contact your GP or healthcare provider if you are currently taking Saxenda and considering adding any over-the-counter weight loss supplements, including Lipozene. Your clinician can review potential interactions, assess whether additional interventions are necessary, and ensure your weight management plan remains safe and effective. Never assume that 'natural' or over-the-counter products are automatically safe to combine with prescription medications.
Seek medical advice promptly if you experience any of the following whilst taking Saxenda, particularly if you have also been using fibre supplements:
-
Severe or persistent abdominal pain, which could indicate pancreatitis (a rare but serious adverse effect of GLP-1 receptor agonists) or gastrointestinal obstruction. Stop taking Saxenda immediately if pancreatitis is suspected.
-
Upper right abdominal pain, fever or jaundice (yellowing of skin/eyes), which could indicate gallbladder problems
-
Difficulty swallowing or sensation of food being stuck in the throat or chest
-
Persistent vomiting that prevents adequate fluid or food intake, increasing the risk of dehydration
-
Signs of dehydration, including dark urine, dizziness, reduced urination, or extreme thirst
-
Severe constipation lasting more than a few days despite usual remedies
-
Unexplained weight loss that exceeds expected rates or occurs with other concerning symptoms
If you are pregnant, planning pregnancy, or breastfeeding, you should not use Saxenda as it is not recommended in these circumstances.
Regular monitoring is essential when using Saxenda. Your specialist or GP should review your progress at appropriate intervals, typically at 12 weeks to assess weight loss response, and subsequently every 3–6 months. These appointments provide opportunities to discuss any concerns, adjust your treatment plan, and ensure you are receiving adequate support for dietary and lifestyle changes.
If you are not currently under medical supervision for weight management but are considering using weight loss medications or supplements, schedule an appointment with your GP. A comprehensive assessment can identify underlying causes of weight gain, screen for contraindications to specific treatments, and develop an individualised management plan. Your GP can also refer you to specialist weight management services (Tier 3), dietitians, or exercise programmes as appropriate. Evidence-based weight management is most successful when delivered as part of a coordinated, multidisciplinary approach rather than through self-directed use of multiple products.
If you experience any suspected side effects from Saxenda, these can be reported through the MHRA Yellow Card scheme, which helps monitor the safety of medicines in the UK.
Frequently Asked Questions
Is it safe to take Saxenda and Lipozene at the same time?
There is no official guidance on combining these products, but both affect gastric emptying and may cause additive gastrointestinal side effects. Always consult your GP or prescribing clinician before combining Saxenda with any weight loss supplements.
What are the main risks of combining Saxenda with glucomannan supplements?
Potential risks include intensified gastrointestinal symptoms (nausea, bloating, constipation), theoretical oesophageal or intestinal obstruction risk, and possible interference with absorption of other oral medications. Adequate fluid intake is essential when using fibre supplements.
Does NICE recommend using dietary supplements alongside Saxenda?
NICE guidelines recommend Saxenda only as an adjunct to lifestyle changes (reduced-calorie diet and increased physical activity) within specialist weight management services. Dietary supplements generally lack the evidence base to be recommended in clinical guidelines.
The health-related content published on this site is based on credible scientific sources and is periodically reviewed to ensure accuracy and relevance. Although we aim to reflect the most current medical knowledge, the material is meant for general education and awareness only.
The information on this site is not a substitute for professional medical advice. For any health concerns, please speak with a qualified medical professional. By using this information, you acknowledge responsibility for any decisions made and understand we are not liable for any consequences that may result.
Heading 1
Heading 2
Heading 3
Heading 4
Heading 5
Heading 6
Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut aliquip ex ea commodo consequat. Duis aute irure dolor in reprehenderit in voluptate velit esse cillum dolore eu fugiat nulla pariatur.
Block quote
Ordered list
- Item 1
- Item 2
- Item 3
Unordered list
- Item A
- Item B
- Item C
Bold text
Emphasis
Superscript
Subscript










