Mounjaro (tirzepatide) is a prescription medicine licensed in the UK for type 2 diabetes and chronic weight management in adults with obesity or overweight with weight-related comorbidities. Accessing Mounjaro through private providers such as Phlo Clinic offers a convenient alternative to NHS pathways, though patients bear the full cost of treatment. Understanding the pricing structure, clinical eligibility criteria, and ongoing financial commitment is essential before commencing therapy. This article examines Mounjaro costs at Phlo Clinic, the consultation process, and key considerations for patients seeking private prescriptions for tirzepatide in the UK.
Summary: Mounjaro costs approximately £150–£250 per month at Phlo Clinic, plus consultation fees of £40–£60 initially and £20–£40 for follow-ups, with total annual costs ranging from £2,000–£3,500.
- Tirzepatide is a dual GIP and GLP-1 receptor agonist administered as a once-weekly subcutaneous injection for type 2 diabetes and chronic weight management.
- NHS access remains limited for weight management, with NICE recommending tirzepatide for adults with BMI ≥35 kg/m² and weight-related comorbidities within specialist services.
- Phlo Clinic provides private prescriptions following online assessment by UK-registered prescribers, with medication delivered directly to patients' homes.
- Common adverse effects include gastrointestinal symptoms such as nausea, vomiting, and diarrhoea, with important safety considerations including pancreatitis and gallbladder disease risk.
- Treatment requires gradual dose titration starting at 2.5 mg weekly, increasing every 4 weeks as tolerated, with ongoing clinical monitoring essential for safety and efficacy.
Table of Contents
What Is Mounjaro and How Does It Work?
Mounjaro (tirzepatide) is a prescription medicine licensed in the UK for the treatment of type 2 diabetes mellitus and, more recently, for chronic weight management in adults with obesity or overweight with weight-related comorbidities. Manufactured by Eli Lilly, Mounjaro is administered as a once-weekly subcutaneous injection and has demonstrated significant efficacy in clinical trials for both glycaemic control and weight reduction.
The drug works through a novel dual mechanism of action. Tirzepatide is a dual glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptor agonist. By activating both GIP and GLP-1 receptors, Mounjaro enhances insulin secretion in a glucose-dependent manner, suppresses glucagon release, slows gastric emptying, and reduces appetite. This dual action distinguishes it from single GLP-1 receptor agonists such as semaglutide (Wegovy, Ozempic).
Clinical studies, including the SURMOUNT and SURPASS trial programmes, have shown that tirzepatide can lead to substantial weight loss—with higher doses achieving up to 15% of baseline body weight in non-diabetic populations—alongside improvements in cardiovascular risk factors such as blood pressure and lipid profiles. Common adverse effects include gastrointestinal symptoms such as nausea, vomiting, diarrhoea, and constipation, which are typically mild to moderate and tend to diminish over time.
Important safety considerations include risk of pancreatitis, gallbladder disease, and hypoglycaemia (particularly when used with insulin or sulphonylureas). Tirzepatide may also cause worsening of diabetic retinopathy in patients with pre-existing retinopathy, particularly with rapid improvement in blood glucose. Dehydration and acute kidney injury can occur secondary to gastrointestinal side effects, so maintaining adequate fluid intake is essential.
Mounjaro is not indicated for type 1 diabetes or diabetic ketoacidosis. It is not recommended during pregnancy or breastfeeding, and women of childbearing potential should use effective contraception. Due to delayed gastric emptying, women using oral contraceptives should consider non-oral or additional barrier methods for 4 weeks after starting treatment and after each dose increase.
Patients should seek urgent medical attention if they experience severe, persistent abdominal pain (which may indicate pancreatitis), signs of allergic reaction, or inability to maintain hydration due to gastrointestinal symptoms.
NHS vs Private Prescriptions: Understanding Your Options
Access to Mounjaro in the UK varies significantly depending on whether you pursue treatment through the NHS or via private healthcare providers. Understanding these pathways is essential for patients considering tirzepatide therapy.
NHS Provision: Currently, Mounjaro is available on the NHS primarily for the treatment of type 2 diabetes in patients who meet specific clinical criteria, as outlined in the product's marketing authorisation and local formulary guidance. For weight management, NHS access remains limited. The National Institute for Health and Care Excellence (NICE) has recently published guidance recommending tirzepatide for chronic weight management in adults with a body mass index (BMI) of ≥35 kg/m² (or ≥32.5 kg/m² in certain ethnic groups) and at least one weight-related comorbidity. Treatment must be initiated and supervised within specialist weight management services, with specific continuation criteria requiring a minimum 5% weight loss at 6 months. However, implementation across NHS trusts is gradual, and availability may be restricted due to supply constraints and local commissioning decisions. Waiting times for specialist services can be considerable.
Private Prescriptions: Many patients opt for private healthcare to access Mounjaro more promptly. Private clinics, including online services such as Phlo Clinic, can assess eligibility and issue private prescriptions following a clinical consultation. This route offers faster access and the ability to commence treatment without NHS waiting lists. However, patients bear the full cost of medication and consultations.
Key Considerations:
-
Eligibility criteria may differ between NHS and private providers, though both should follow appropriate clinical guidelines.
-
Private prescriptions require out-of-pocket payment, and NHS GPs are not obliged to continue prescribing medication initiated privately.
-
Patients should ensure any private provider is regulated by the Care Quality Commission (CQC) and employs registered healthcare professionals.
-
It is advisable to inform your NHS GP of any privately prescribed medications to ensure coordinated care and monitoring of potential side effects or interactions.
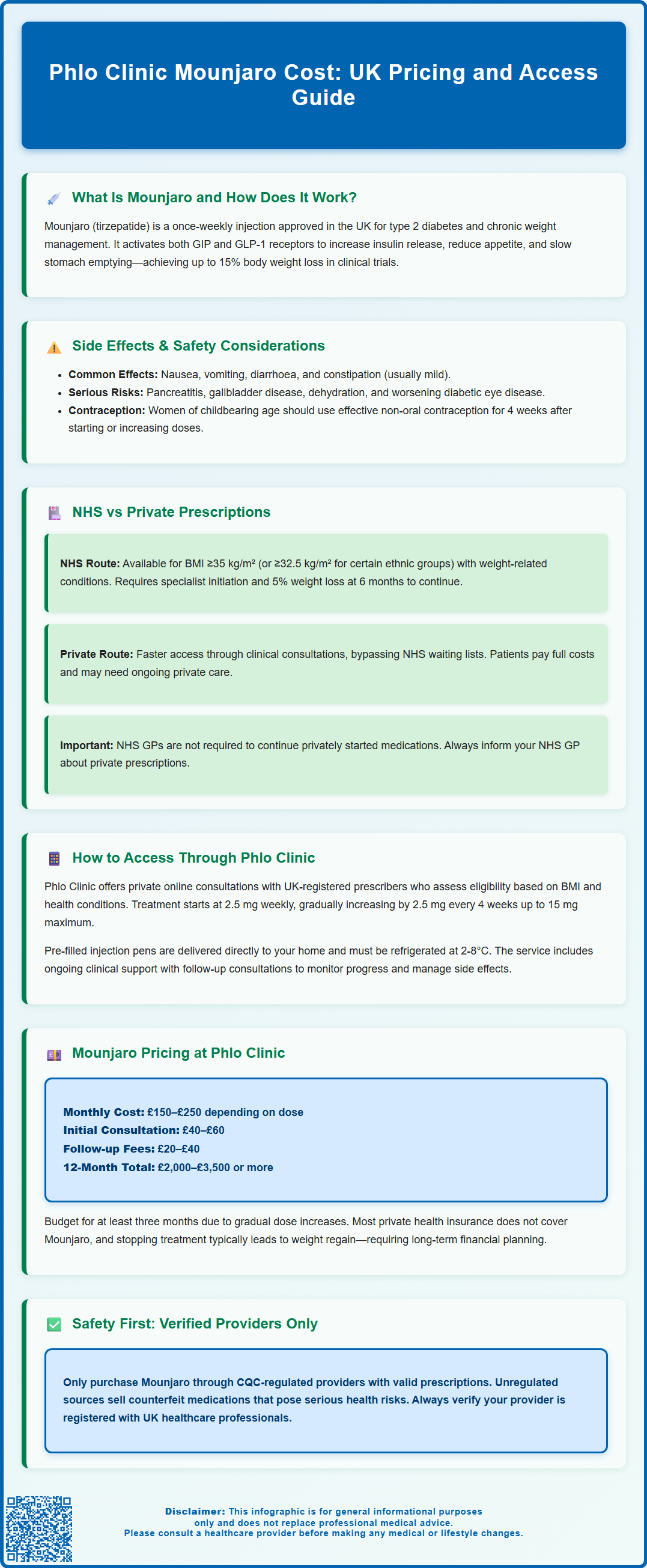
How to Access Mounjaro Through Phlo Clinic
Phlo Clinic is a UK-based online healthcare service offering private consultations and prescriptions for weight management treatments, including Mounjaro. The service is designed to provide convenient access to tirzepatide for eligible patients who prefer private care.
Step-by-Step Process:
1. Online Assessment: Patients begin by completing a comprehensive online medical questionnaire on the Phlo Clinic website. This assessment gathers information about medical history, current medications, weight management goals, previous weight loss attempts, and any contraindications to tirzepatide therapy. Accurate and honest disclosure is essential to ensure patient safety.
2. Clinical Review: A UK-registered prescriber—typically a GP or independent prescriber—reviews the submitted information. The clinician assesses eligibility based on clinical guidelines, including BMI thresholds, presence of weight-related comorbidities (such as hypertension, type 2 diabetes, dyslipidaemia, or obstructive sleep apnoea), and absence of contraindications. If further information is required, the clinician may contact the patient directly.
3. Prescription Issuance: If deemed suitable, the prescriber issues a private prescription for Mounjaro. The prescription specifies the starting dose (typically 2.5 mg once weekly) and outlines the titration schedule. Standard titration follows the UK product guidance: 2.5 mg weekly for 4 weeks, then increasing by 2.5 mg every 4 weeks as tolerated (to 5 mg, 7.5 mg, 10 mg, 12.5 mg, and potentially 15 mg).
4. Medication Delivery: Phlo Clinic arranges delivery of the medication directly to the patient's home address. Mounjaro is supplied in pre-filled, single-use injection pens and requires refrigeration (2-8°C) upon receipt. The medication should not be frozen, should be protected from light, and can be kept at room temperature for up to 21 days. Patients receive instructions on storage, administration technique (including suitable injection sites), and arrangements for safe disposal of used pens via a sharps bin.
5. Ongoing Support: Phlo Clinic provides ongoing clinical support, including follow-up consultations to monitor progress, manage side effects, and adjust dosing as needed. Baseline and periodic monitoring of weight, blood pressure, and relevant blood tests (such as renal function, HbA1c if diabetic) is recommended. Patients are encouraged to maintain regular contact with their NHS GP for comprehensive health monitoring.
Mounjaro Cost at Phlo Clinic: Pricing and Payment Options
The cost of Mounjaro through private providers such as Phlo Clinic reflects the medication's price, consultation fees, and service charges. Understanding the financial commitment is crucial for patients considering this treatment pathway.
Indicative Pricing Structure:
The following figures are indicative only and should be confirmed directly with Phlo Clinic, as prices may vary and are subject to change:
-
Initial Consultation Fee: Approximately £40–£60 for the online assessment and prescriber review.
-
Monthly Medication Cost: Mounjaro typically costs between £150–£250 per month, depending on the prescribed dose. Lower doses (2.5 mg, 5 mg) are generally less expensive than higher maintenance doses (10 mg, 15 mg).
-
Follow-Up Consultations: Ongoing clinical reviews may incur additional fees, often around £20–£40 per consultation.
Patients should budget for a minimum three-month commitment, as tirzepatide requires gradual dose titration and sustained use to achieve meaningful weight loss. Over a 12-month treatment course, total costs may range from £2,000–£3,500 or more, depending on the final maintenance dose and frequency of clinical reviews.
Payment Options:
Phlo Clinic typically offers:
-
One-off payments for each prescription cycle (usually monthly supply).
-
Subscription models that may provide continuity of supply.
-
Payment via debit/credit card or other secure online methods.
Important Financial Considerations:
-
Mounjaro is not currently reimbursed by private health insurance in most cases, though policies vary—patients should check with their insurer.
-
Treatment is an ongoing expense; clinical evidence from the SURMOUNT-4 trial indicates that discontinuation typically leads to weight regain, so long-term financial planning is essential.
-
Patients should compare costs across multiple private providers and ensure transparency regarding all fees.
-
If financial constraints arise, discuss options with your prescriber, including potential transition to NHS services if eligibility criteria are met.
Patient Safety Reminder: Never purchase Mounjaro from unregulated online sources or without a valid prescription. Counterfeit or improperly stored medications pose serious health risks. Always verify that your provider is CQC-registered and employs qualified healthcare professionals. Report any suspected side effects to the Medicines and Healthcare products Regulatory Agency (MHRA) through the Yellow Card Scheme.
Scientific References
- Mounjaro KwikPen 2.5mg solution for injection in pre-filled pen - Summary of Product Characteristics (SmPC).
- Mounjaro - European Public Assessment Report (EPAR).
- Tirzepatide for managing overweight and obesity. Technology Appraisal TA1026.
- Tirzepatide Once Weekly for the Treatment of Obesity (SURMOUNT-1).
- Tirzepatide versus Semaglutide Once Weekly in Patients with Type 2 Diabetes (SURPASS-2).
- Obesity - Treatment.
Frequently Asked Questions
How much does Mounjaro cost at Phlo Clinic per month?
Mounjaro typically costs between £150–£250 per month at Phlo Clinic, depending on the prescribed dose, with lower doses (2.5 mg, 5 mg) generally less expensive than higher maintenance doses (10 mg, 15 mg). Initial consultation fees are approximately £40–£60, with follow-up consultations costing around £20–£40.
Is Mounjaro available on the NHS for weight management?
NHS access to Mounjaro for weight management remains limited. NICE recommends tirzepatide for adults with BMI ≥35 kg/m² (or ≥32.5 kg/m² in certain ethnic groups) and at least one weight-related comorbidity, but treatment must be initiated within specialist weight management services with specific continuation criteria requiring minimum 5% weight loss at 6 months.
What is the process for getting Mounjaro through Phlo Clinic?
Patients complete an online medical questionnaire, which is reviewed by a UK-registered prescriber who assesses eligibility based on clinical guidelines. If suitable, a private prescription is issued and the medication is delivered directly to the patient's home, with ongoing clinical support provided for monitoring and dose adjustments.
The health-related content published on this site is based on credible scientific sources and is periodically reviewed to ensure accuracy and relevance. Although we aim to reflect the most current medical knowledge, the material is meant for general education and awareness only.
The information on this site is not a substitute for professional medical advice. For any health concerns, please speak with a qualified medical professional. By using this information, you acknowledge responsibility for any decisions made and understand we are not liable for any consequences that may result.
Heading 1
Heading 2
Heading 3
Heading 4
Heading 5
Heading 6
Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut aliquip ex ea commodo consequat. Duis aute irure dolor in reprehenderit in voluptate velit esse cillum dolore eu fugiat nulla pariatur.
Block quote
Ordered list
- Item 1
- Item 2
- Item 3
Unordered list
- Item A
- Item B
- Item C
Bold text
Emphasis
Superscript
Subscript












