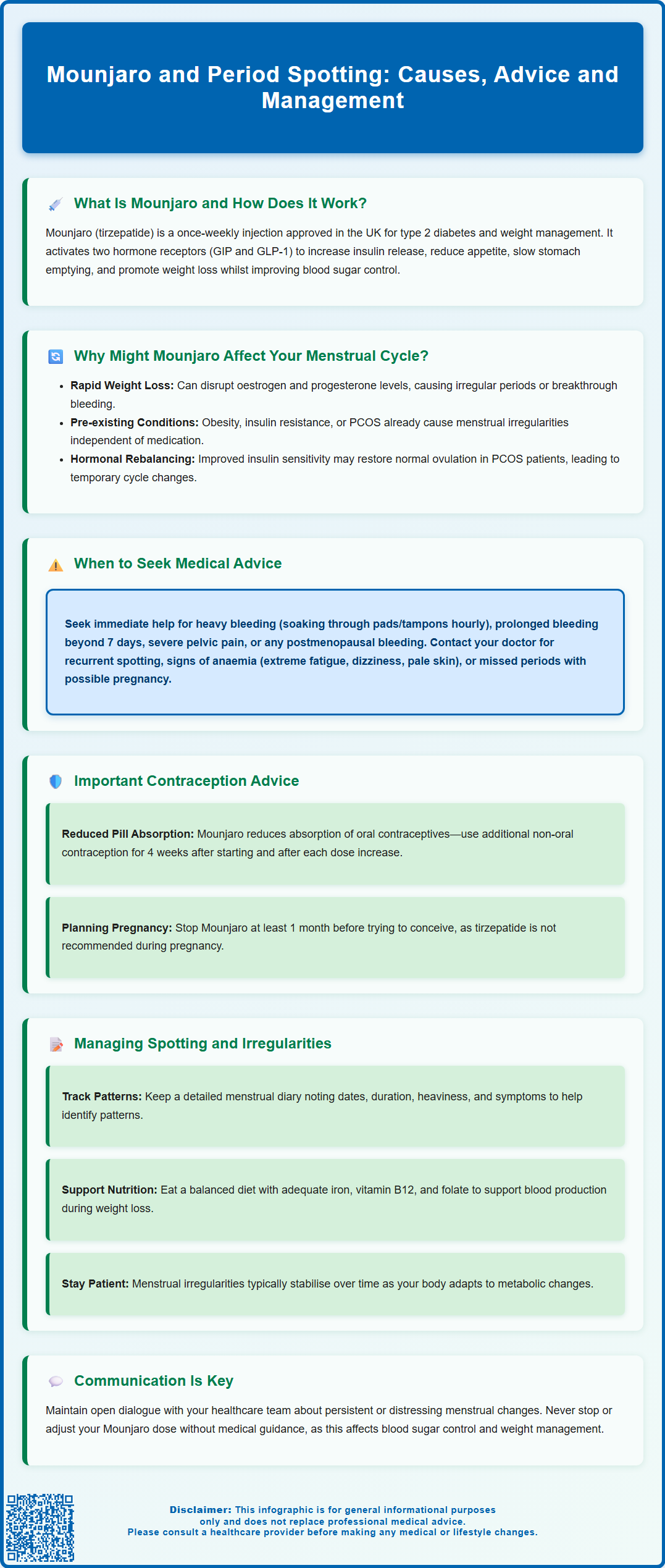
Mounjaro (tirzepatide) is a dual GIP/GLP-1 receptor agonist licensed in the UK for type 2 diabetes and weight management. Whilst Mounjaro and period spotting are not officially linked in prescribing information, some individuals report menstrual changes after starting treatment. These alterations may relate to rapid weight loss, hormonal shifts, or improved metabolic function rather than direct medication effects. Understanding the potential mechanisms, recognising when to seek medical advice, and knowing how to manage symptoms are essential for anyone experiencing menstrual irregularities whilst taking Mounjaro. This article explores the evidence, clinical considerations, and practical guidance for patients and healthcare professionals.
Summary: Mounjaro (tirzepatide) is not officially linked to period spotting, but menstrual changes may occur indirectly through rapid weight loss and hormonal shifts affecting the hypothalamic-pituitary-ovarian axis.
- Tirzepatide is a dual GIP/GLP-1 receptor agonist licensed for type 2 diabetes and weight management in the UK.
- Rapid weight loss can alter oestrogen and progesterone levels, potentially causing irregular periods or breakthrough bleeding.
- Pre-existing conditions such as PCOS may improve with weight loss, leading to changes in menstrual patterns.
- Seek urgent medical advice for heavy bleeding, prolonged bleeding, severe pain, or postmenopausal bleeding.
- Mounjaro reduces oral contraceptive absorption; use additional barrier contraception for 4 weeks after starting and after each dose increase.
- Most menstrual irregularities associated with weight loss stabilise over time as the body adapts to metabolic changes.
Table of Contents
What Is Mounjaro and How Does It Work?
Mounjaro (tirzepatide) is a prescription medicine licensed in the UK for the treatment of type 2 diabetes and, more recently, for weight management in adults with obesity or overweight with weight-related comorbidities. It belongs to a class of medications known as dual glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptor agonists. By activating both GIP and GLP-1 receptors, Mounjaro enhances insulin secretion in response to meals, suppresses glucagon release, slows gastric emptying, and reduces appetite—leading to improved glycaemic control and weight reduction.
The medication is administered once weekly via subcutaneous injection, typically in the abdomen, thigh, or upper arm. Dosing begins at 2.5 mg weekly and is gradually increased over several weeks (2.5→5→7.5→10→12.5→15 mg) to minimise gastrointestinal side effects, with maintenance doses ranging from 5 mg to 15 mg depending on individual response and tolerability. NICE guidance supports its use in specific patient populations where other treatments have proven inadequate.
Common adverse effects include nausea, vomiting, diarrhoea, constipation, abdominal pain, and decreased appetite—most of which are mild to moderate and tend to diminish over time. Serious but rare risks include pancreatitis, gallbladder disease, and hypoglycaemia (particularly when used with insulin or sulfonylureas). Patients are advised to report persistent abdominal pain, jaundice, or signs of severe allergic reactions immediately. If you experience any suspected side effects, you can report them via the MHRA Yellow Card scheme. Understanding how Mounjaro works systemically is important when considering its potential effects on other bodily functions, including the menstrual cycle.
Why Might Mounjaro Affect Your Menstrual Cycle?
While menstrual irregularities and spotting are not listed as direct side effects in the official Mounjaro prescribing information, there are several plausible biological mechanisms through which tirzepatide may indirectly influence the menstrual cycle. The most significant factor is rapid weight loss, which is a common effect of GLP-1 and dual GIP/GLP-1 receptor agonists. Substantial or rapid reduction in body weight can alter the hypothalamic-pituitary-ovarian (HPO) axis, leading to changes in oestrogen and progesterone levels. This hormonal shift may result in irregular periods, breakthrough bleeding, or spotting between cycles.
Additionally, many individuals starting Mounjaro have pre-existing metabolic conditions such as obesity, insulin resistance, or polycystic ovary syndrome (PCOS)—all of which are independently associated with menstrual irregularities. Weight loss and improved insulin sensitivity can actually restore ovulatory function in some individuals with PCOS, potentially leading to changes in cycle regularity or unexpected bleeding as the body adjusts to a more normal hormonal environment.
There is limited research suggesting whether GLP-1 receptor agonists might have direct effects on reproductive tissues. Some animal studies suggest GLP-1 receptors are present in ovarian and uterine tissues, but the clinical significance in humans is not yet established. It is important to note that there is no official link confirmed between Mounjaro and menstrual spotting in regulatory documentation, and any changes should be evaluated individually.
Other contributing factors may include stress, dietary changes, or concurrent medications. If you experience new or unusual menstrual symptoms after starting Mounjaro, it is advisable to discuss these with your GP or healthcare professional to rule out other causes.
When to Seek Medical Advice About Period Changes on Mounjaro
While some menstrual changes may be expected during significant weight loss or metabolic improvement, certain symptoms warrant prompt medical evaluation. You should contact your GP or healthcare professional if you experience:
-
Heavy bleeding (soaking through a pad or tampon every hour for several consecutive hours) or passing large clots
-
Prolonged bleeding lasting more than seven days or bleeding that does not stop
-
Severe pelvic or abdominal pain that is new, persistent, or worsening
-
Postmenopausal bleeding (any vaginal bleeding occurring 12 months or more after your last period) – this requires an urgent 2-week suspected cancer referral
-
Bleeding between periods that is recurrent, increasing in frequency, or accompanied by other concerning symptoms
-
Signs of anaemia, such as extreme fatigue, dizziness, shortness of breath, or pale skin
It is also important to seek advice if you have missed periods and there is any possibility of pregnancy. Mounjaro affects gastric emptying, which can reduce the absorption of oral contraceptives. According to the UK product information, if you use oral contraceptives, you should use additional or alternative non-oral contraception for 4 weeks after starting Mounjaro and for 4 weeks after each dose increase. If you are of childbearing potential and sexually active, a pregnancy test should be performed to exclude pregnancy, as tirzepatide is not recommended during pregnancy. You should stop tirzepatide at least 1 month before planned conception.
Additionally, if you have a history of gynaecological conditions such as endometriosis, fibroids, or previous abnormal cervical screening results, any new bleeding pattern should be evaluated more thoroughly. Your GP may recommend a pelvic examination, blood tests, transvaginal ultrasound, or referral to gynaecology depending on your individual risk factors and clinical presentation. Do not assume that spotting is simply a side effect of Mounjaro without proper assessment, as it may indicate an unrelated but significant gynaecological issue requiring investigation.
Managing Spotting and Menstrual Irregularities While Taking Mounjaro
If you experience mild spotting or menstrual changes that have been assessed by your healthcare professional and deemed non-concerning, there are several practical strategies to help manage these symptoms while continuing Mounjaro therapy.
Monitoring and documentation are essential. Keep a menstrual diary recording the dates, duration, and heaviness of bleeding or spotting episodes, along with any associated symptoms such as cramping or mood changes. This information is invaluable for your healthcare team in determining whether patterns are stabilising or require further investigation. Many smartphone apps can facilitate this tracking.
Lifestyle and nutritional support play an important role, particularly during periods of significant weight loss. Ensure you are consuming a balanced, nutrient-rich diet with adequate iron, vitamin B12, folate, and other micronutrients to support healthy blood production and hormonal function. If you are experiencing heavy or frequent bleeding, your GP may recommend iron supplementation or blood tests to check for anaemia.
Maintain open communication with your diabetes or weight management team. If menstrual irregularities are distressing or persistent, your prescriber may consider adjusting the dose escalation schedule or evaluating whether Mounjaro remains the most appropriate treatment option for you. However, do not stop or alter your Mounjaro dose without medical guidance, as this may affect your glycaemic control or weight management progress.
For individuals with PCOS or other underlying hormonal conditions, menstrual changes during Mounjaro treatment may reflect improving metabolic health. Your GP or endocrinologist can help differentiate between expected physiological adjustments and symptoms requiring intervention. In some cases, hormonal contraception may be recommended to regulate cycles, though this decision should be made collaboratively with your healthcare professional considering your overall health goals. If using oral contraceptives, remember that you need additional barrier contraception or a non-oral method for 4 weeks after starting Mounjaro and for 4 weeks after each dose increase.
It's important to note that weight loss may improve fertility, particularly in women with PCOS, so reliable contraception is essential if pregnancy is not desired.
Finally, be reassured that for most individuals, menstrual irregularities associated with weight loss tend to stabilise over time as the body adapts to a new metabolic equilibrium. Patience, monitoring, and regular follow-up are key to ensuring both your metabolic and reproductive health remain optimally managed.
Frequently Asked Questions
Can Mounjaro cause period spotting or irregular bleeding?
Mounjaro is not officially linked to period spotting, but menstrual changes may occur indirectly through rapid weight loss and hormonal shifts. If you experience new or concerning bleeding patterns, consult your GP to rule out other causes.
When should I see a doctor about menstrual changes on Mounjaro?
Seek medical advice for heavy bleeding (soaking through pads hourly), prolonged bleeding beyond seven days, severe pelvic pain, postmenopausal bleeding, or signs of anaemia such as extreme fatigue or dizziness.
Does Mounjaro affect contraception and fertility?
Mounjaro reduces absorption of oral contraceptives, so use additional barrier contraception for 4 weeks after starting and after each dose increase. Weight loss may improve fertility, particularly in women with PCOS, so reliable contraception is essential if pregnancy is not desired.
The health-related content published on this site is based on credible scientific sources and is periodically reviewed to ensure accuracy and relevance. Although we aim to reflect the most current medical knowledge, the material is meant for general education and awareness only.
The information on this site is not a substitute for professional medical advice. For any health concerns, please speak with a qualified medical professional. By using this information, you acknowledge responsibility for any decisions made and understand we are not liable for any consequences that may result.
Heading 1
Heading 2
Heading 3
Heading 4
Heading 5
Heading 6
Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut aliquip ex ea commodo consequat. Duis aute irure dolor in reprehenderit in voluptate velit esse cillum dolore eu fugiat nulla pariatur.
Block quote
Ordered list
- Item 1
- Item 2
- Item 3
Unordered list
- Item A
- Item B
- Item C
Bold text
Emphasis
Superscript
Subscript












