Rybelsus (semaglutide) is an oral medication licensed in the UK for treating type 2 diabetes mellitus in adults. As a glucagon-like peptide-1 (GLP-1) receptor agonist, it mimics a naturally occurring hormone that regulates blood glucose levels. Understanding how Rybelsus lowers blood sugar can help patients and healthcare professionals optimise treatment outcomes. This article explains the mechanism of action, expected glucose reductions, time to effect, and factors influencing treatment response. Rybelsus works through multiple pathways to improve glycaemic control whilst reducing the risk of hypoglycaemia compared to some other diabetes medications.
Summary: Rybelsus lowers blood sugar by activating GLP-1 receptors, which stimulates glucose-dependent insulin secretion, suppresses glucagon release, and slows gastric emptying.
- Rybelsus is an oral GLP-1 receptor agonist licensed for type 2 diabetes treatment in UK adults.
- It stimulates insulin secretion only when blood glucose is elevated, reducing hypoglycaemia risk compared to some other diabetes medications.
- Clinical trials show HbA1c reductions of approximately 1.0–1.4% with the 14 mg maintenance dose after 26 weeks.
- Full therapeutic effect typically becomes apparent after 12–16 weeks of treatment at a stable dose.
- Must be taken on waking with up to 120 mL water, at least 30 minutes before food or other medications, and swallowed whole.
- Regular monitoring and adherence to dosing instructions are essential for optimal glycaemic control.
Table of Contents
What Is Rybelsus and How Does It Work?
Rybelsus (semaglutide) is an oral medication licensed in the UK for the treatment of type 2 diabetes mellitus in adults. It belongs to a class of drugs called glucagon-like peptide-1 (GLP-1) receptor agonists, which mimic the action of a naturally occurring hormone in the body that helps regulate blood glucose levels.
The mechanism by which Rybelsus lowers blood sugar is multifaceted. When you eat, your intestines release GLP-1, a hormone that signals the pancreas to produce insulin in response to rising glucose levels. Semaglutide works by binding to and activating GLP-1 receptors throughout the body, particularly in the pancreas. This activation stimulates insulin secretion from pancreatic beta cells, but importantly, this effect is glucose-dependent—meaning insulin is only released when blood sugar levels are elevated, which reduces the risk of hypoglycaemia (low blood sugar) compared to some other diabetes medications. However, the risk of hypoglycaemia increases when Rybelsus is used with a sulfonylurea or insulin, and dose reductions of these medications may be needed.
Additionally, Rybelsus suppresses the release of glucagon, a hormone that normally raises blood glucose by triggering the liver to release stored glucose. By reducing glucagon secretion when it is not needed, Rybelsus helps prevent excessive glucose production by the liver. The medication also slows gastric emptying, meaning food moves more slowly from the stomach into the small intestine. This results in a more gradual rise in blood sugar after meals and can contribute to increased feelings of fullness, which may support modest weight management—though Rybelsus is not licensed for weight loss.
Rybelsus is typically prescribed as monotherapy when metformin is unsuitable, or as a combination therapy with other diabetes medications when adequate glycaemic control has not been achieved. It is available in three tablet strengths (3 mg, 7 mg, and 14 mg) and must be taken correctly for proper absorption: on waking with up to 120 mL of water, at least 30 minutes before any food, drink, or other oral medicines. The tablet must be swallowed whole—not split, crushed or chewed. Rybelsus should not be used in type 1 diabetes, diabetic ketoacidosis, or concomitantly with other GLP-1 receptor agonists.
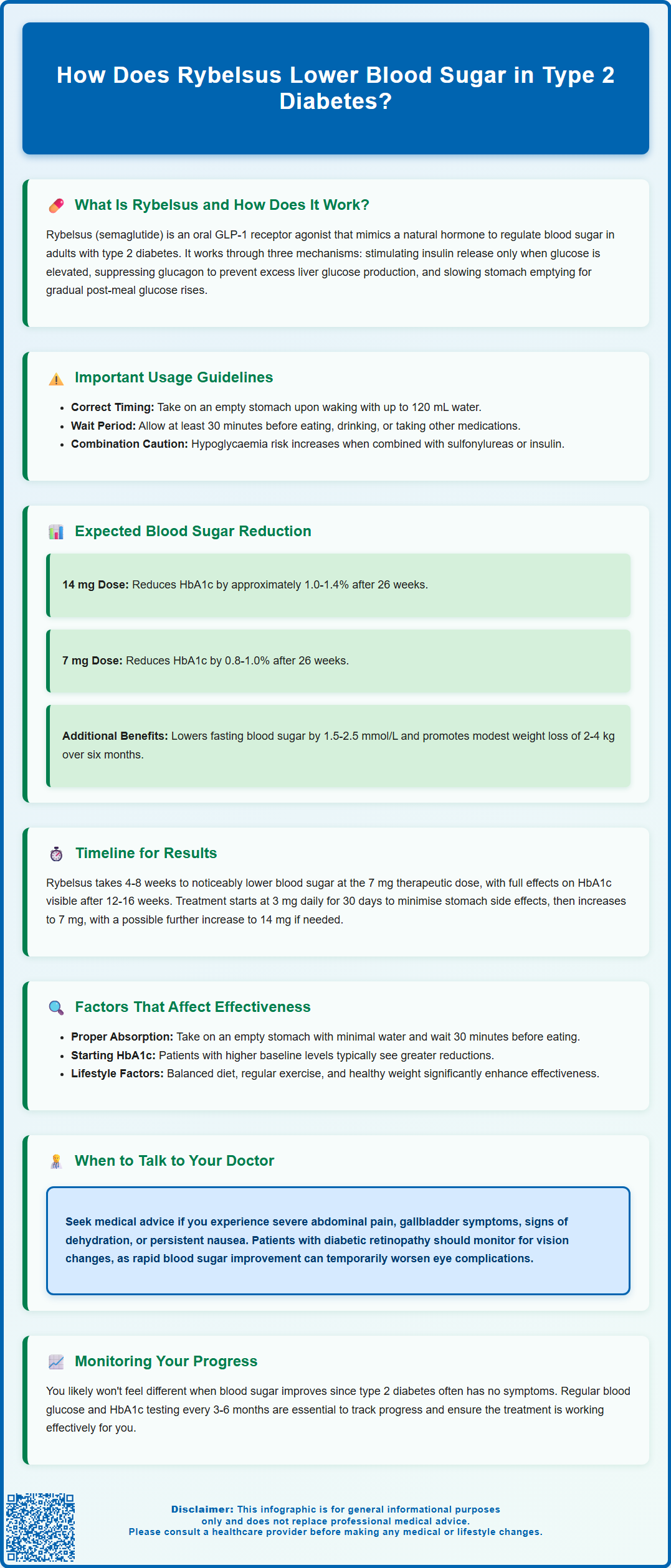
Expected Blood Sugar Reduction with Rybelsus
Clinical trials have demonstrated that Rybelsus produces clinically meaningful reductions in HbA1c (glycated haemoglobin), the key measure of long-term blood glucose control. HbA1c reflects average blood sugar levels over the previous two to three months, and lowering this value reduces the risk of diabetes-related complications, particularly microvascular outcomes such as kidney damage and neuropathy.
In the PIONEER clinical trial programme, patients taking Rybelsus 14 mg (the maximum maintenance dose) experienced an average HbA1c reduction of approximately 1.0% to 1.4% from baseline after 26 weeks of treatment. Those on the 7 mg dose typically saw reductions of around 0.8% to 1.0%. These results were observed when Rybelsus was used either as monotherapy or in combination with other diabetes medications such as metformin, sulfonylureas, or SGLT2 inhibitors.
For context, NICE guidelines recommend aiming for an HbA1c target of 48 mmol/mol (6.5%) or lower for most adults with type 2 diabetes who are managed with a drug not associated with hypoglycaemia. For those on medications associated with hypoglycaemia risk (such as sulfonylureas or insulin), a target of 53 mmol/mol (7.0%) may be more appropriate. Rybelsus can help many patients achieve or move closer to these targets, particularly when baseline HbA1c is elevated.
Beyond HbA1c, Rybelsus also improves fasting plasma glucose (blood sugar measured after an overnight fast) and postprandial glucose (blood sugar levels after meals). Reductions in fasting glucose of 1.5 to 2.5 mmol/L have been reported in clinical trials. Additionally, many patients experience modest weight loss (typically 2 to 4 kg over six months), which can further improve insulin sensitivity and glycaemic control, though Rybelsus is not licensed for weight management. It is important to note that individual responses vary, and not all patients will achieve the same degree of blood sugar reduction. Regular monitoring and follow-up with your healthcare team are essential to assess treatment effectiveness.
How Long Does Rybelsus Take to Lower Blood Sugar?
The time course for blood sugar reduction with Rybelsus follows a gradual and progressive pattern. Unlike some diabetes medications that produce immediate effects, GLP-1 receptor agonists require time to reach steady-state concentrations in the body and exert their full therapeutic effect. Semaglutide has a half-life of approximately one week, with steady-state concentrations achieved after 4-5 weeks of consistent dosing.
Patients typically begin Rybelsus at a starting dose of 3 mg once daily for the first 30 days. This initial dose is primarily intended to improve gastrointestinal tolerability rather than to achieve maximum glucose lowering. Some patients may notice modest improvements in blood sugar during this first month, but significant reductions are generally not expected at this dose.
After 30 days, the dose is usually increased to 7 mg once daily. At this therapeutic dose, most patients begin to see noticeable improvements in blood glucose levels within 4 to 8 weeks. However, the full effect on HbA1c—which reflects longer-term glucose control—typically becomes apparent after 12 to 16 weeks of treatment at a stable dose. This is because HbA1c represents the average blood sugar over the preceding two to three months, so it takes time for improvements in daily glucose levels to be reflected in this measure.
For patients who require further glucose lowering, the dose may be increased to 14 mg once daily after at least 30 days on the 7 mg dose. Again, the maximum benefit at this higher dose is usually observed after several months of consistent use.
If you miss a dose, skip the missed dose and take your next dose the following day. Do not take a double dose to make up for a missed dose.
It is important to continue taking Rybelsus as prescribed even if you do not notice immediate changes in how you feel. Type 2 diabetes is often asymptomatic, and improvements in blood sugar may not produce obvious physical sensations. Regular blood glucose monitoring and periodic HbA1c testing (typically every 3 to 6 months) are the most reliable ways to assess treatment response. People with pre-existing diabetic retinopathy should be aware that rapid improvement in blood glucose control may temporarily worsen retinopathy; discuss any visual changes with your diabetes care team. If adequate glycaemic control is not achieved after several months at the maximum tolerated dose, your GP or diabetes specialist may recommend adding or switching to alternative therapies.
Factors That Affect How Well Rybelsus Works
Several factors can influence the effectiveness of Rybelsus in lowering blood sugar, and understanding these can help optimise treatment outcomes.
Adherence to dosing instructions is critical. Rybelsus must be taken on an empty stomach with no more than 120 mL of water, and you must wait at least 30 minutes before eating, drinking, or taking other oral medications. The tablet must be swallowed whole—not split, crushed or chewed. Failure to follow these instructions can significantly reduce absorption and diminish the drug's effectiveness. Taking Rybelsus at the same time each morning can help establish a consistent routine.
Baseline HbA1c and disease duration also play a role. Patients with higher starting HbA1c levels often experience greater absolute reductions, though they may still require combination therapy to reach target levels. Conversely, those with longer-standing type 2 diabetes and reduced pancreatic beta-cell function may have a more limited response to GLP-1 agonists, as these medications rely on the presence of functioning insulin-producing cells.
Concomitant medications can affect outcomes. Rybelsus is often used alongside metformin, which works through a different mechanism and can provide additive glucose-lowering effects. Rybelsus should not be used with other GLP-1 receptor agonists. If you take warfarin, more frequent INR monitoring may be needed. Levothyroxine levels may also require monitoring when starting Rybelsus.
Lifestyle factors remain foundational. A balanced diet, regular physical activity, and weight management all enhance the effectiveness of Rybelsus. Conversely, poor dietary choices, sedentary behaviour, and weight gain can counteract the medication's benefits. Gastrointestinal side effects—particularly nausea, which affects up to 20% of patients—can sometimes limit dose escalation or lead to discontinuation, thereby reducing overall effectiveness. If you experience severe or persistent abdominal pain (which could indicate pancreatitis), symptoms of gallbladder disease, or signs of dehydration, seek prompt medical advice.
No dose adjustment is required for patients with renal or hepatic impairment, though caution is advised in severe impairment or end-stage renal disease. Patients experiencing significant gastrointestinal symptoms should maintain adequate fluid intake to prevent dehydration and acute kidney injury.
Finally, individual variability in drug metabolism and receptor sensitivity means that response to Rybelsus can differ between patients. If you have concerns about how well Rybelsus is working for you, or if you experience side effects that affect your ability to take the medication as prescribed, contact your GP or diabetes care team. They can assess your response, adjust your treatment plan, and ensure you receive appropriate support to achieve optimal blood sugar control. Report any suspected side effects to the MHRA Yellow Card Scheme (yellowcard.mhra.gov.uk).
Frequently Asked Questions
How quickly does Rybelsus start lowering blood sugar?
Most patients begin to see noticeable improvements in blood glucose levels within 4 to 8 weeks of starting the 7 mg therapeutic dose. However, the full effect on HbA1c typically becomes apparent after 12 to 16 weeks of treatment at a stable dose.
Can Rybelsus cause low blood sugar (hypoglycaemia)?
Rybelsus has a lower risk of hypoglycaemia when used alone because it stimulates insulin release only when blood glucose is elevated. However, the risk increases when combined with sulfonylureas or insulin, and dose reductions of these medications may be needed.
What happens if I don't take Rybelsus correctly on an empty stomach?
Failure to take Rybelsus on an empty stomach with no more than 120 mL of water, or eating within 30 minutes of dosing, can significantly reduce drug absorption and diminish its effectiveness in lowering blood sugar.
The health-related content published on this site is based on credible scientific sources and is periodically reviewed to ensure accuracy and relevance. Although we aim to reflect the most current medical knowledge, the material is meant for general education and awareness only.
The information on this site is not a substitute for professional medical advice. For any health concerns, please speak with a qualified medical professional. By using this information, you acknowledge responsibility for any decisions made and understand we are not liable for any consequences that may result.
Heading 1
Heading 2
Heading 3
Heading 4
Heading 5
Heading 6
Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut aliquip ex ea commodo consequat. Duis aute irure dolor in reprehenderit in voluptate velit esse cillum dolore eu fugiat nulla pariatur.
Block quote
Ordered list
- Item 1
- Item 2
- Item 3
Unordered list
- Item A
- Item B
- Item C
Bold text
Emphasis
Superscript
Subscript












