Rybelsus (semaglutide) is an oral medication for type 2 diabetes mellitus, supplied in the UK in aluminium blister packs rather than bottles. This specialised packaging protects the moisture-sensitive tablets, ensuring they remain effective. Understanding how to correctly access and take Rybelsus from its blister packaging is essential for optimal glycaemic control. This guide explains the proper technique for removing tablets from the blister pack, the critical administration requirements, and when to seek professional support if you encounter difficulties with your medication.
Summary: Rybelsus is supplied in aluminium blister packs in the UK, not bottles, and tablets are removed by pressing through or peeling back the foil backing immediately before use.
- Rybelsus contains semaglutide, a GLP-1 receptor agonist for type 2 diabetes mellitus treatment
- Tablets must remain in original blister packaging until use to protect against moisture degradation
- Take on an empty stomach with up to 120 ml water, waiting 30 minutes before eating or taking other medicines
- Available in three strengths: 3 mg, 7 mg, and 14 mg tablets
- Pharmacists can demonstrate opening techniques or provide blister-aid devices for patients with reduced dexterity
- Contact your GP or diabetes team if you experience persistent side effects or difficulty managing the medication
Table of Contents
Understanding Rybelsus Packaging Design
Rybelsus (semaglutide) is an oral medication licensed in the UK for the treatment of type 2 diabetes mellitus. In the UK, Rybelsus is supplied in aluminium/aluminium blister packs, not bottles. This specific packaging is designed to protect the tablets from moisture, which is critical as semaglutide is sensitive to environmental conditions that could compromise its efficacy.
The Rybelsus blister packaging is specifically engineered to maintain the stability of the medication. Each tablet is individually sealed within the blister, creating a protective barrier against moisture and other environmental factors that could affect the medicine's potency.
Key features of Rybelsus packaging include:
-
Aluminium/aluminium blister packs for moisture protection
-
Individual tablet sealing to maintain integrity
-
Clear labelling with dosage strength (3 mg, 7 mg, or 14 mg)
-
Tamper-evident packaging
It is essential to keep Rybelsus tablets in their original blister packaging until you are ready to take them. The tablets should never be removed and stored in pill organisers or dosette boxes, as this would expose them to moisture and potentially reduce their effectiveness.
Individuals with reduced hand strength or dexterity, such as those with arthritis, may find blister packs challenging to open. If you experience difficulties, your pharmacist can demonstrate techniques for opening the blister pack or suggest suitable blister-aid devices.
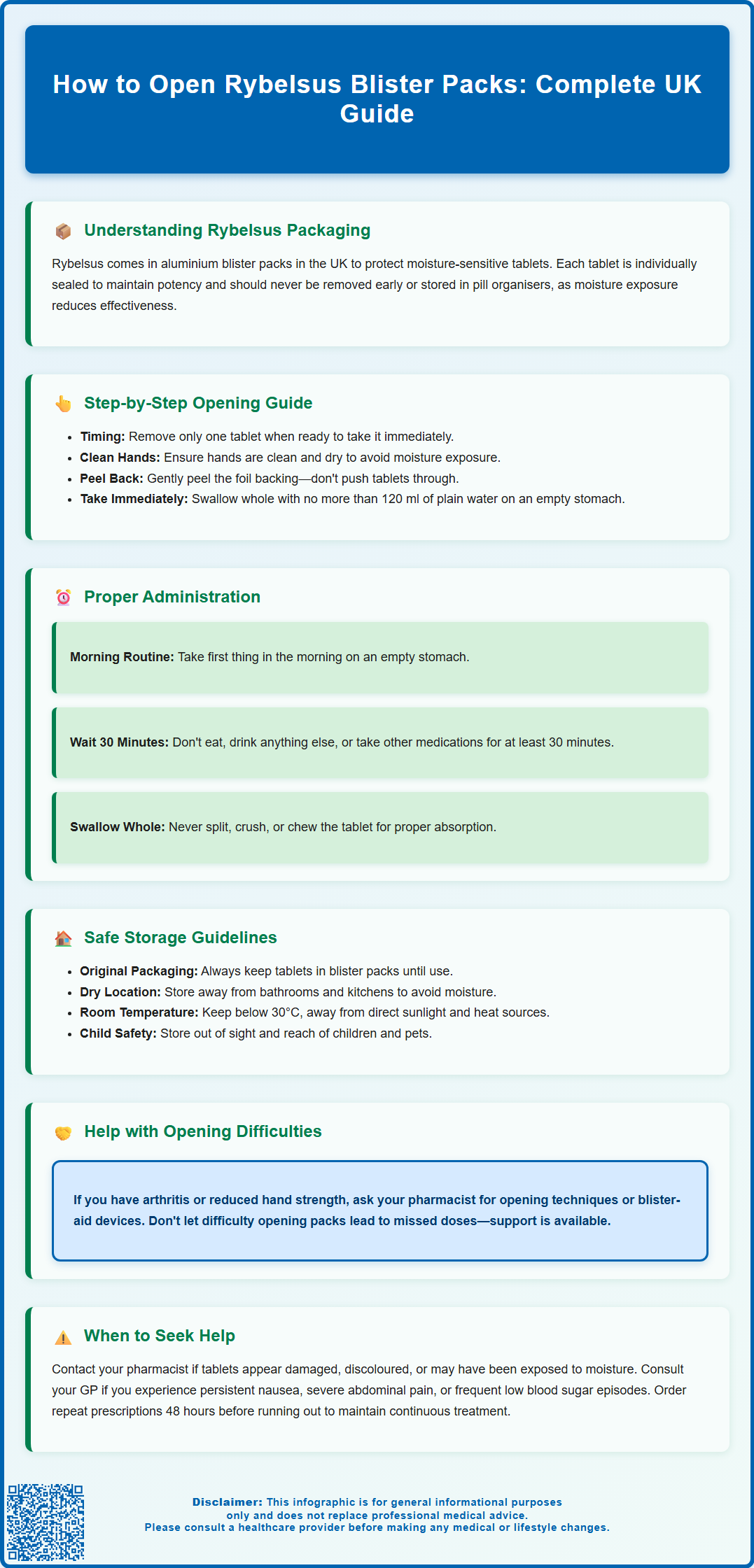
Step-by-Step Guide to Taking Rybelsus from the Blister Pack
Taking Rybelsus requires following specific steps to ensure you access your medication safely and take it correctly for maximum effectiveness. Before attempting to open the blister pack, ensure your hands are clean and dry, as moisture can affect the medication.
Step 1: Prepare to take your tablet Rybelsus should be taken when you first wake up, on an empty stomach, with no more than 120 ml (a small glass) of water. This timing is crucial for proper absorption.
Step 2: Remove a single tablet from the blister Place the blister pack on a flat, stable surface. Carefully press the tablet through the foil backing or peel back the foil if indicated on the packaging. Only remove one tablet when you are ready to take it immediately.
Step 3: Take the tablet correctly Swallow the tablet whole with up to 120 ml of plain water. Do not split, crush, or chew the tablet as this can affect how the medicine works.
Step 4: Wait before eating or taking other medicines After taking Rybelsus, you must wait at least 30 minutes before consuming any food, drink, or other oral medicines. This waiting period is essential for proper absorption of the medication.
Step 5: Store remaining tablets properly Keep the remaining tablets in their original blister packaging in a dry place. Do not remove tablets until you are ready to take them, as exposure to air and moisture can reduce their effectiveness.
Common Issues When Taking Rybelsus and How to Resolve Them
Patients occasionally encounter difficulties when using their Rybelsus medication, particularly if they have conditions affecting hand function. Understanding these common challenges and their solutions can help ensure uninterrupted access to your medication.
Difficulty opening blister packs: Some patients find it challenging to press tablets through the foil backing or manipulate the blister packaging, especially those with arthritis, reduced grip strength, or other conditions affecting manual dexterity. If you struggle with this, speak with your pharmacist who can demonstrate techniques for opening blister packs or recommend suitable blister-aid devices designed to assist with this task.
Remembering the administration routine: The specific requirements for taking Rybelsus (on an empty stomach with water, followed by a 30-minute wait) can be difficult to incorporate into daily routines. Setting an alarm 30 minutes before your usual breakfast time can help establish a consistent pattern. Some patients find it helpful to keep the medication and a glass for water by their bedside to take immediately upon waking.
Damaged or broken tablets: If a tablet appears damaged when removed from the blister or breaks during removal, do not take it. Consult your pharmacist about proper disposal and taking your next dose.
Forgetting the 30-minute waiting period: The waiting period after taking Rybelsus is crucial for proper absorption. If you accidentally eat or drink (other than water) within this 30-minute window, the effectiveness of the medication may be reduced. Make a note of this for your next diabetes review but continue with your normal dosing schedule.
If problems persist despite these strategies, contact your diabetes care team to discuss whether Rybelsus remains the most suitable treatment option for you, or if additional support strategies might be helpful.
Storing Rybelsus Safely
Proper storage of Rybelsus is crucial to maintain the medication's efficacy and ensure patient safety. Semaglutide tablets are particularly sensitive to environmental conditions, and incorrect storage can compromise their therapeutic effect.
Keep tablets in the original blister packaging: Always store Rybelsus tablets in their original blister packaging until immediately before use. Do not transfer them to pill organisers, dosette boxes, or alternative containers, as this exposes them to moisture and removes them from the protective environment of the sealed blister. The original packaging is specifically designed to maintain optimal conditions for the medication.
Protect from moisture: Moisture is the primary environmental factor that can degrade Rybelsus tablets. Store the blister packs in a dry location away from bathrooms, kitchens, or other areas with high humidity. Ensure tablets remain in their intact blister cells until you are ready to take them—even brief exposure to air can allow moisture ingress.
Temperature considerations: Store Rybelsus at room temperature, not above 30°C as specified in the UK product information. Avoid exposure to direct sunlight or heat sources such as radiators or windowsills. Store in a cool, dry place, following the specific storage instructions on the packaging or patient information leaflet.
Child safety and security: Store the medication in a location that is out of sight and reach of children and pets. Consider using a locked medicine cabinet if young children frequently visit your home.
Shelf life: Rybelsus should be used before the expiry date printed on the packaging. If you notice any changes in tablet appearance, such as discolouration or unusual odour, do not use the medication and consult your pharmacist.
Regularly check your medication supply to ensure you do not run out unexpectedly, as Rybelsus requires consistent daily dosing for optimal glycaemic control.
When to Seek Help with Your Rybelsus Prescription
There are several circumstances in which you should contact your healthcare provider, pharmacist, or GP regarding your Rybelsus prescription. Recognising when professional guidance is needed ensures both medication safety and therapeutic effectiveness.
Persistent difficulty with blister packs: If you consistently struggle to open your Rybelsus blister packaging, contact your community pharmacist. They can provide a practical demonstration or suggest suitable blister-aid devices. Do not allow difficulty with packaging to result in missed doses, as consistent daily administration is essential for glycaemic control.
Concerns about medication integrity: If you suspect your Rybelsus tablets have been exposed to moisture—for example, if tablets were removed from the blister in advance or the tablets appear different from usual—consult your pharmacist before taking them. Compromised tablets may have reduced efficacy. Similarly, if tablets have been stored incorrectly (such as in excessive heat or humidity), seek professional advice about whether they remain suitable for use.
Running low on medication: Contact your GP surgery or use the NHS repeat prescription service well before your current supply runs out. Rybelsus requires continuous daily dosing, and interruptions in treatment can affect your diabetes management. Most surgeries require at least 48 hours' notice for repeat prescriptions, though this varies by practice.
Adverse effects or concerns about treatment: If you experience side effects such as persistent nausea, vomiting, abdominal pain, or signs of pancreatitis (severe upper abdominal pain radiating to the back), contact your GP promptly. Other important symptoms to report include signs of dehydration from vomiting or diarrhoea, visual changes (which could indicate worsening diabetic retinopathy), or symptoms of gallstones. You can also report any suspected side effects directly to the MHRA through the Yellow Card scheme (yellowcard.mhra.gov.uk or the Yellow Card app).
Risk of hypoglycaemia: If you take Rybelsus alongside a sulfonylurea medication or insulin, you may have an increased risk of low blood sugar (hypoglycaemia). Monitor your blood glucose levels as advised by your healthcare team and seek medical review if you experience frequent or severe hypoglycaemic episodes.
Damaged or defective packaging: If your Rybelsus blister pack arrives damaged, with broken seals, or appears to have been tampered with, do not use the medication. Return it to your pharmacy immediately for a replacement.
Your healthcare team is available to support you with both practical aspects of medication use and clinical concerns. Never hesitate to seek guidance—proper medication management is fundamental to achieving optimal outcomes in type 2 diabetes treatment.
Frequently Asked Questions
Does Rybelsus come in a bottle in the UK?
No, Rybelsus is supplied in aluminium blister packs in the UK, not bottles. This moisture-protective packaging is essential to maintain the medication's stability and effectiveness.
Can I remove Rybelsus tablets in advance and store them in a pill organiser?
No, you should never remove Rybelsus tablets from their blister packaging until immediately before taking them. Exposure to moisture can reduce the medication's effectiveness, so tablets must remain in their protective blister until use.
What should I do if I struggle to open Rybelsus blister packs?
Contact your community pharmacist who can demonstrate proper opening techniques or recommend blister-aid devices. These tools are particularly helpful for patients with arthritis or reduced hand strength.
The health-related content published on this site is based on credible scientific sources and is periodically reviewed to ensure accuracy and relevance. Although we aim to reflect the most current medical knowledge, the material is meant for general education and awareness only.
The information on this site is not a substitute for professional medical advice. For any health concerns, please speak with a qualified medical professional. By using this information, you acknowledge responsibility for any decisions made and understand we are not liable for any consequences that may result.
Heading 1
Heading 2
Heading 3
Heading 4
Heading 5
Heading 6
Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut aliquip ex ea commodo consequat. Duis aute irure dolor in reprehenderit in voluptate velit esse cillum dolore eu fugiat nulla pariatur.
Block quote
Ordered list
- Item 1
- Item 2
- Item 3
Unordered list
- Item A
- Item B
- Item C
Bold text
Emphasis
Superscript
Subscript












