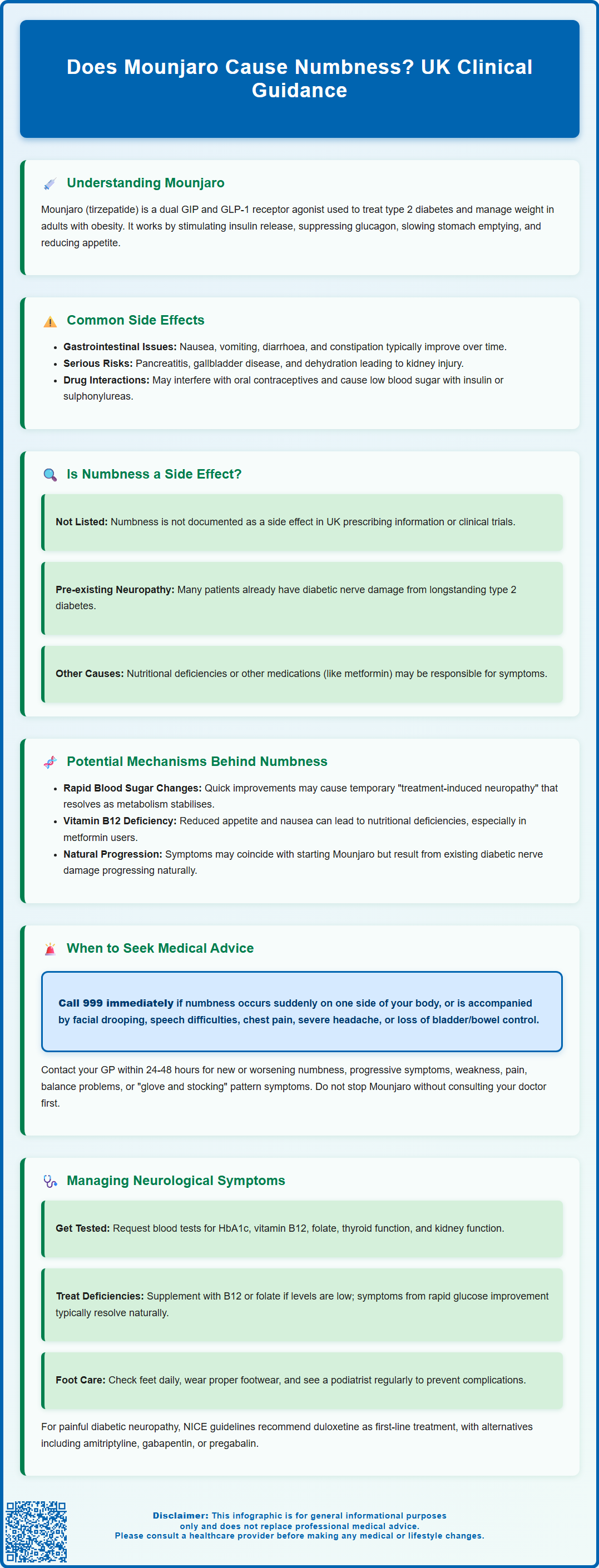
Does Mounjaro cause numbness? This question concerns many patients prescribed tirzepatide for type 2 diabetes or weight management. Numbness and tingling (paraesthesia) are not listed as recognised adverse reactions in the UK Summary of Product Characteristics for Mounjaro. However, patients may experience these symptoms for various reasons during treatment, including pre-existing diabetic neuropathy, rapid metabolic changes, nutritional deficiencies, or concurrent medications. Understanding the distinction between direct medication effects and alternative causes is essential for appropriate clinical assessment and management.
Summary: Numbness is not a recognised adverse reaction to Mounjaro (tirzepatide) according to UK regulatory documentation and clinical trial data.
- Mounjaro is a dual GIP and GLP-1 receptor agonist licensed in the UK for type 2 diabetes and weight management in adults with obesity or overweight with comorbidities.
- Common side effects predominantly affect the gastrointestinal system, including nausea, vomiting, diarrhoea, constipation, and abdominal discomfort.
- Numbness during treatment may result from pre-existing diabetic neuropathy, rapid glycaemic improvement, nutritional deficiencies (particularly vitamin B12), or concurrent medications.
- Patients experiencing new, worsening, or progressive numbness should seek medical assessment within 24–48 hours; sudden onset with stroke symptoms requires immediate emergency care (call 999).
- Management requires comprehensive clinical assessment including neurological examination, blood tests for vitamin deficiencies and metabolic control, and investigation of alternative causes before attributing symptoms to Mounjaro.
Table of Contents
Understanding Mounjaro and Its Common Side Effects
Mounjaro (tirzepatide) is a prescription medicine licensed in the UK for the treatment of type 2 diabetes mellitus and for weight management in adults with obesity or overweight with weight-related comorbidities. It belongs to a novel class of medications known as dual glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptor agonists. By mimicking the action of these naturally occurring incretin hormones, Mounjaro helps regulate blood glucose levels, reduces appetite, and slows gastric emptying.
The mechanism of action involves binding to both GIP and GLP-1 receptors, which stimulates insulin secretion in a glucose-dependent manner, suppresses glucagon release, and promotes satiety through central nervous system pathways. This dual action distinguishes tirzepatide from single GLP-1 receptor agonists and contributes to its efficacy in glycaemic control and weight reduction.
Common side effects associated with Mounjaro predominantly affect the gastrointestinal system. According to the MHRA/EMC Summary of Product Characteristics (SmPC), these include:
-
Nausea and vomiting – often most pronounced during dose escalation
-
Diarrhoea or constipation – affecting bowel regularity
-
Abdominal pain and discomfort – typically mild to moderate
-
Reduced appetite – a therapeutic effect that may feel uncomfortable initially
-
Dyspepsia – indigestion or heartburn
These gastrointestinal effects usually diminish over time as the body adjusts to treatment. Less common but notable adverse effects include injection site reactions, fatigue, and hypoglycaemia (particularly when used alongside insulin or sulfonylureas).
The SmPC also notes serious adverse effects including pancreatitis, gallbladder disease, risk of dehydration and acute kidney injury, and potential worsening of diabetic retinopathy (particularly in patients with pre-existing retinopathy when glycaemic control improves rapidly). The delayed gastric emptying effect may affect absorption of some oral medicines, including oral contraceptives.
Understanding the expected side effect profile helps patients and clinicians distinguish between typical treatment responses and symptoms requiring further investigation.
Can Mounjaro Cause Numbness or Tingling?
Numbness and tingling sensations (medically termed paraesthesia) are not listed as adverse reactions in the UK SmPC for Mounjaro. The SURPASS clinical trial programme, which evaluated tirzepatide's safety and efficacy across thousands of participants, did not identify peripheral neuropathy or paraesthesia as a significant adverse event attributable to the medication, as summarised in the European Medicines Agency's European Public Assessment Report (EPAR).
When patients experience numbness whilst taking Mounjaro, alternative explanations should be thoroughly explored:
-
Pre-existing diabetic neuropathy – many patients prescribed Mounjaro have longstanding type 2 diabetes, a condition frequently complicated by peripheral nerve damage
-
Rapid metabolic changes – significant improvements in glycaemic control may temporarily affect nerve function
-
Nutritional factors – reduced food intake due to gastrointestinal symptoms or appetite suppression might affect nutrient intake
-
Concurrent medications – polypharmacy in diabetes management may include drugs with neurological side effects, particularly metformin which can reduce vitamin B12 levels with long-term use (MHRA Drug Safety Update)
Whilst individual case reports exist in patient forums and anecdotal accounts, these do not constitute clinical evidence of causation. Healthcare professionals should approach reports of numbness during Mounjaro treatment with appropriate clinical investigation rather than automatically attributing symptoms to the medication. A comprehensive assessment of alternative causes remains essential for accurate diagnosis and appropriate management.
Potential Mechanisms Behind Numbness During Mounjaro Treatment
When patients experience numbness whilst taking Mounjaro, understanding the potential underlying mechanisms helps guide appropriate investigation and management. Although not a recognised direct effect of tirzepatide, several physiological processes associated with its use might theoretically contribute to neurological symptoms.
Rapid glycaemic improvement represents one plausible mechanism. When blood glucose levels decrease substantially and quickly, some patients experience a phenomenon called "treatment-induced neuropathy of diabetes" or acute painful diabetic neuropathy. This paradoxical worsening of neuropathic symptoms can occur when glucose control improves rapidly after a period of poor control. The sudden metabolic shift may temporarily disrupt nerve function before longer-term benefits emerge. This condition typically improves as metabolic stability is achieved.
Nutritional factors may develop secondary to Mounjaro's effects on appetite and digestion. Persistent nausea, reduced appetite, and altered eating patterns can compromise intake of essential nutrients. Vitamin B12 deficiency is particularly important to consider, especially in patients also taking metformin, which is known to reduce B12 levels with long-term use (MHRA Drug Safety Update). B12 deficiency causes peripheral neuropathy characterised by numbness and tingling in the extremities. Folate levels may also be relevant in some cases.
Diabetic peripheral neuropathy progression remains the most common explanation for numbness in this patient population. Many individuals prescribed Mounjaro have established diabetes with existing nerve damage that may progress independently of treatment. The temporal association with starting Mounjaro may be coincidental rather than causal.
Distinguishing between these possibilities requires careful clinical assessment, including neurological examination, metabolic screening, and consideration of the patient's diabetes history and overall health status.
When to Seek Medical Advice About Numbness
Patients experiencing numbness or tingling whilst taking Mounjaro should understand when to contact their GP or diabetes specialist for assessment. Whilst not all neurological symptoms require urgent attention, certain features warrant prompt medical evaluation.
Seek medical advice within 24–48 hours if you experience:
-
New or worsening numbness affecting the hands, feet, or other body areas
-
Progressive symptoms that spread or intensify over days or weeks
-
Accompanying weakness in the affected limbs or difficulty with fine motor tasks
-
Pain or burning sensations alongside numbness
-
Balance problems or difficulty walking that may indicate more extensive nerve involvement
-
Symptoms affecting both sides of the body symmetrically, particularly in a "glove and stocking" distribution
-
Any foot problems including ulcers, infections, or changes in colour/temperature with numbness
Seek urgent medical attention if numbness is accompanied by:
-
Sudden onset affecting one side of the body – call 999 or go to A&E immediately (potential stroke warning)
-
Facial drooping, speech difficulties, or visual changes – call 999 (use the FAST test: Face, Arms, Speech, Time)
-
Chest pain, severe headache, or confusion – call 999 or go to A&E
-
Loss of bladder or bowel control (potential spinal cord involvement) – go to A&E
-
Severe weakness or inability to move affected limbs – go to A&E
-
Foot ulceration, infection or ischaemia with neuropathy – seek same-day assessment from your GP or multidisciplinary foot care service
For non-urgent but persistent symptoms, arrange a routine GP appointment for comprehensive assessment. Your healthcare provider will need to:
-
Review your diabetes history and current glycaemic control
-
Examine neurological function systematically
-
Assess for vitamin deficiencies, particularly B12 (especially if you take metformin)
-
Evaluate medication history and potential drug interactions
-
Consider whether symptoms represent diabetic neuropathy progression
Do not stop taking Mounjaro without medical advice, as abrupt discontinuation may affect diabetes control. Your doctor can determine whether symptoms relate to the medication or require investigation for alternative causes. If you're concerned about a possible side effect, you can report it via the MHRA Yellow Card Scheme (yellowcard.mhra.gov.uk or the Yellow Card app).
Managing and Monitoring Neurological Symptoms on Mounjaro
Effective management of numbness or neurological symptoms during Mounjaro treatment requires a systematic approach combining investigation, monitoring, and appropriate interventions based on identified causes.
Initial clinical assessment should include a thorough neurological examination to characterise the distribution, severity, and nature of symptoms. Your healthcare provider will likely arrange blood tests to evaluate:
-
HbA1c and glucose profiles – to assess glycaemic control and recent changes
-
Vitamin B12 levels – particularly important if you take metformin
-
Folate levels – to exclude deficiency that may contribute to neuropathy
-
Thyroid function – as hypothyroidism can cause peripheral neuropathy
-
Renal function – to exclude uraemic neuropathy
-
Full blood count – to identify anaemia or other haematological issues
Depending on findings, further investigations such as nerve conduction studies or referral to neurology may be warranted for persistent, severe or atypical symptoms.
Management strategies depend on the underlying cause:
For nutritional deficiencies: Supplementation with vitamin B12 (oral or intramuscular) or folate alongside dietary optimisation and management of gastrointestinal symptoms.
For rapid glycaemic improvement: Reassurance and monitoring, as treatment-induced neuropathy typically improves spontaneously over time. Maintaining stable glucose control prevents further episodes.
For diabetic neuropathy: NICE guidelines recommend optimising glycaemic control as the primary intervention. For painful diabetic neuropathy, NICE recommends duloxetine as first-line treatment. If duloxetine is contraindicated, not tolerated or ineffective, consider amitriptyline, gabapentin, or pregabalin as alternatives.
Foot care is essential for patients with neuropathy. Perform daily foot checks, wear appropriate footwear, and attend regular podiatry appointments. Any signs of foot ulceration, infection or ischaemia with neuropathy require urgent referral to a multidisciplinary foot care service.
Ongoing monitoring should include regular review of symptoms, repeat neurological examination, and reassessment of metabolic control. If symptoms persist or worsen despite investigation and management of identified causes, discuss with your diabetes specialist whether continuing Mounjaro remains appropriate or whether alternative treatments should be considered. Most importantly, maintain open communication with your healthcare team, report any changes promptly, and attend scheduled follow-up appointments to ensure comprehensive care and optimal outcomes during your treatment journey.
Frequently Asked Questions
Is numbness a recognised side effect of Mounjaro?
No, numbness (paraesthesia) is not listed as an adverse reaction in the UK Summary of Product Characteristics for Mounjaro, nor was it identified as a significant adverse event in the SURPASS clinical trial programme.
What causes numbness in patients taking Mounjaro?
Numbness during Mounjaro treatment is typically caused by pre-existing diabetic neuropathy, rapid improvements in blood glucose control, nutritional deficiencies (especially vitamin B12), or concurrent medications rather than the tirzepatide itself.
When should I see a doctor about numbness whilst taking Mounjaro?
Seek medical advice within 24–48 hours for new, worsening, or progressive numbness, particularly if accompanied by weakness, pain, or balance problems. Call 999 immediately if numbness occurs suddenly on one side of the body with facial drooping or speech difficulties, as these may indicate stroke.
The health-related content published on this site is based on credible scientific sources and is periodically reviewed to ensure accuracy and relevance. Although we aim to reflect the most current medical knowledge, the material is meant for general education and awareness only.
The information on this site is not a substitute for professional medical advice. For any health concerns, please speak with a qualified medical professional. By using this information, you acknowledge responsibility for any decisions made and understand we are not liable for any consequences that may result.
Heading 1
Heading 2
Heading 3
Heading 4
Heading 5
Heading 6
Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut aliquip ex ea commodo consequat. Duis aute irure dolor in reprehenderit in voluptate velit esse cillum dolore eu fugiat nulla pariatur.
Block quote
Ordered list
- Item 1
- Item 2
- Item 3
Unordered list
- Item A
- Item B
- Item C
Bold text
Emphasis
Superscript
Subscript












