Does Mounjaro cause fatigue? Mounjaro (tirzepatide) is a dual GLP-1 and GIP receptor agonist licensed in the UK for type 2 diabetes and weight management. According to the MHRA-approved Summary of Product Characteristics, fatigue is listed as a common side effect, potentially affecting up to 1 in 10 people. Tiredness may result from reduced caloric intake, blood glucose changes, gastrointestinal symptoms, or the body's adjustment to metabolic shifts. This article examines why fatigue occurs with Mounjaro, how to manage it effectively, and when to seek medical advice, providing evidence-based guidance aligned with UK clinical practice.
Summary: Fatigue is a common side effect of Mounjaro (tirzepatide), affecting up to 1 in 10 people according to MHRA data.
- Tirzepatide is a dual GLP-1 and GIP receptor agonist licensed for type 2 diabetes and weight management in the UK.
- Fatigue may result from reduced caloric intake, blood glucose fluctuations, gastrointestinal side effects, or metabolic adaptation to weight loss.
- Management includes maintaining adequate nutrition and hydration, monitoring blood glucose levels, and gradual dose escalation as prescribed.
- Seek medical advice if fatigue is severe, persistent, or accompanied by hypoglycaemia, dehydration, severe abdominal pain, or cardiovascular symptoms.
- Hypoglycaemia risk increases when Mounjaro is combined with insulin or sulfonylureas, requiring dose adjustments by healthcare professionals.
- Regular monitoring and individualised treatment adjustments are essential components of safe Mounjaro use according to NICE guidance.
Table of Contents
Does Mounjaro Cause Fatigue?
Mounjaro (tirzepatide) is a prescription medication licensed in the UK for the treatment of type 2 diabetes mellitus and, more recently, for weight management in adults with obesity or overweight with weight-related comorbidities, as per NICE guidance. As a glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) receptor agonist, it works by mimicking naturally occurring hormones that regulate blood sugar levels and appetite.
According to the MHRA-approved Summary of Product Characteristics (SmPC), fatigue is listed as a common side effect of Mounjaro, meaning it may affect up to 1 in 10 people. The most frequently reported side effects include gastrointestinal symptoms such as nausea, diarrhoea, vomiting, constipation, and abdominal discomfort. Some patients do report experiencing tiredness or low energy levels whilst taking this medication.
It is important to recognise that fatigue can arise from multiple factors when starting or adjusting to Mounjaro. These may include changes in dietary intake due to reduced appetite, alterations in blood glucose levels, the body's adjustment period to the medication, or underlying health conditions. Additionally, rapid weight loss—a desired therapeutic outcome for many patients—can sometimes be accompanied by temporary feelings of tiredness as the body adapts to metabolic changes.
If you are experiencing persistent or concerning fatigue whilst taking Mounjaro, it is essential to discuss this with your GP or diabetes specialist nurse. They can assess whether the tiredness is related to the medication, your overall health status, or other contributing factors, and provide appropriate guidance tailored to your individual circumstances.
It's worth noting that Mounjaro is not indicated for the treatment of type 1 diabetes or diabetic ketoacidosis.
Why Fatigue May Occur with Mounjaro
Understanding the potential mechanisms behind fatigue in patients taking Mounjaro requires consideration of both the medication's pharmacological effects and the broader context of metabolic changes. Tirzepatide's mechanism of action involves activating GLP-1 and GIP receptors, which enhance insulin secretion in a glucose-dependent manner, suppress glucagon release, slow gastric emptying, and reduce appetite. These effects collectively improve glycaemic control and promote weight loss.
Reduced caloric intake is one of the primary reasons patients may experience tiredness. Mounjaro significantly decreases appetite, leading many individuals to consume fewer calories than their body has been accustomed to. This caloric deficit, whilst beneficial for weight loss, can initially result in feelings of low energy or fatigue as the body adjusts to operating on reduced fuel intake. Ensuring adequate nutrition within the context of reduced appetite becomes particularly important.
Blood glucose fluctuations may also contribute to fatigue. For patients with type 2 diabetes, improved glycaemic control can sometimes lead to relative hypoglycaemia—blood sugar levels that, whilst not dangerously low, are lower than what the body has adapted to over time. This adjustment period can manifest as tiredness. Hypoglycaemia risk is increased primarily when Mounjaro is used in combination with insulin or sulfonylureas, as noted in the MHRA SmPC.
Gastrointestinal side effects such as nausea, vomiting, or diarrhoea—which are common with Mounjaro—can lead to dehydration, electrolyte imbalances, and reduced nutrient absorption. These factors can independently cause or exacerbate feelings of tiredness. Additionally, rapid weight loss itself places metabolic demands on the body, and the physiological adaptation to significant weight reduction can temporarily affect energy levels.
Other potential causes of fatigue in patients with type 2 diabetes should also be considered, including anaemia and vitamin B12 deficiency (particularly in those taking metformin), as well as pre-existing conditions, concurrent medications, or lifestyle factors rather than Mounjaro itself.
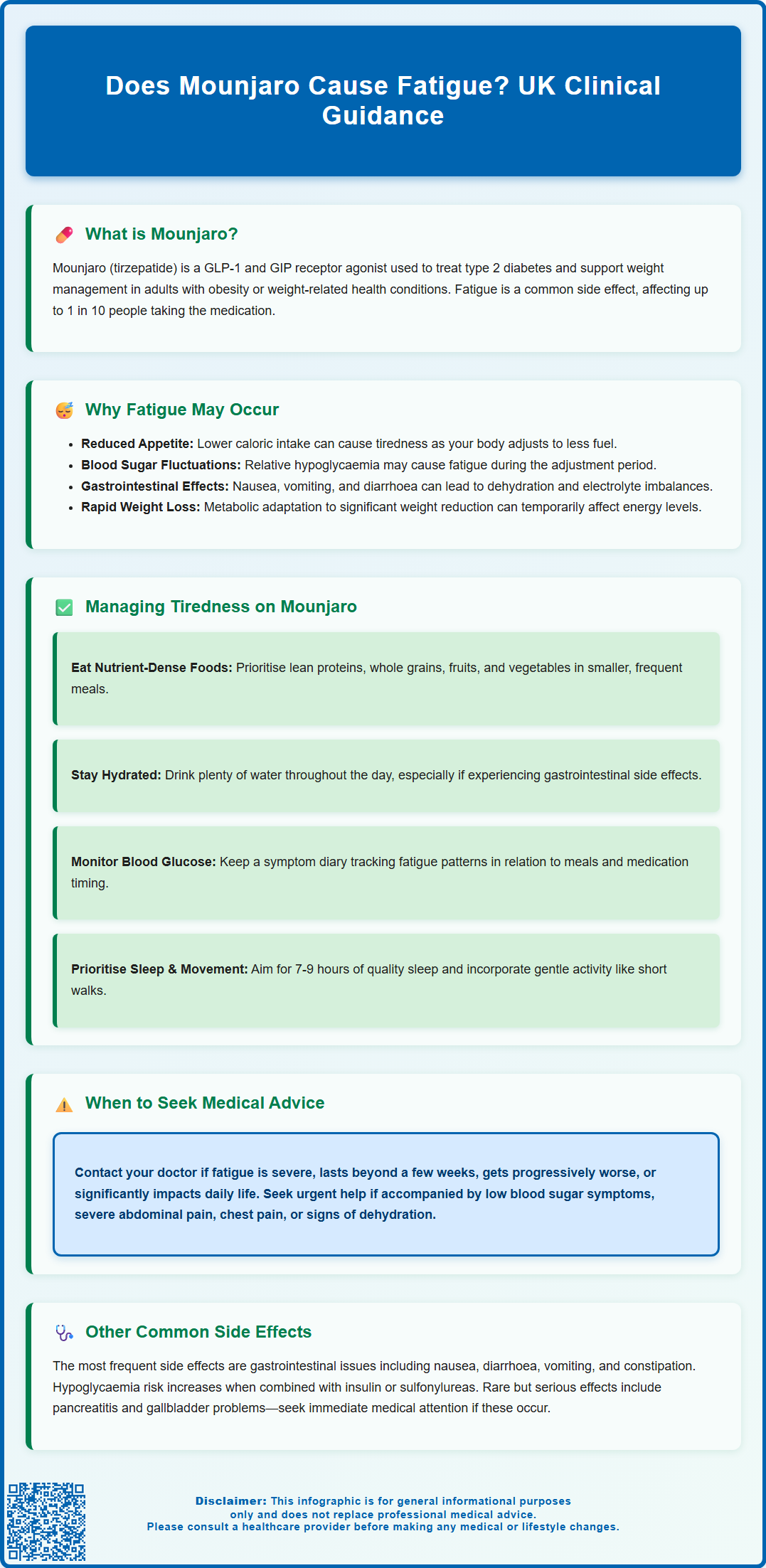
Managing Tiredness While Taking Mounjaro
If you are experiencing fatigue whilst taking Mounjaro, several practical strategies may help manage this symptom whilst continuing to benefit from the medication's therapeutic effects. Maintaining adequate nutrition is paramount, even with reduced appetite. Focus on consuming nutrient-dense foods that provide essential vitamins, minerals, and macronutrients. Prioritise lean proteins, whole grains, fruits, vegetables, and healthy fats. Eating smaller, more frequent meals throughout the day may be easier to manage than three large meals, particularly if nausea is present. Choosing bland, lower-fat meals may help improve gastrointestinal tolerance.
Hydration is equally important, especially if you are experiencing gastrointestinal side effects. Aim to drink adequate fluids throughout the day—water is ideal, but oral rehydration solutions may be beneficial if you have had significant vomiting or diarrhoea. Dehydration can significantly contribute to feelings of tiredness and can be easily overlooked.
Monitoring blood glucose levels as advised by your healthcare team helps identify any patterns of hypoglycaemia or significant fluctuations that might be contributing to fatigue. This is particularly important if you are taking insulin or sulfonylureas alongside Mounjaro, as your doctor may need to adjust dosages to prevent low blood sugar episodes. Keep a symptom diary noting when fatigue occurs in relation to meals, medication timing, and blood glucose readings—this information can be valuable for your healthcare provider.
Lifestyle modifications can also support energy levels. Ensure you are getting adequate sleep (7–9 hours for most adults), as poor sleep quality can compound feelings of tiredness. Gentle physical activity, even short walks, can paradoxically improve energy levels and support overall wellbeing, though it is important to listen to your body and not overexert yourself.
Gradual dose escalation of Mounjaro, as prescribed by your doctor (starting at 2.5 mg weekly and titrating at intervals of at least 4 weeks), allows your body time to adjust to the medication, potentially minimising side effects including fatigue. Never adjust your dose without medical guidance. A review of your other medications with your healthcare provider may also be beneficial.
When to Seek Medical Advice About Fatigue
Whilst mild tiredness during the initial weeks of Mounjaro treatment may be expected as your body adjusts, certain situations warrant prompt medical attention. Contact your GP or diabetes care team if fatigue is severe, persistent beyond the first few weeks of treatment, or progressively worsening. Fatigue that significantly interferes with your daily activities, work, or quality of life should not be dismissed as a normal side effect.
Seek urgent medical advice if fatigue is accompanied by:
-
Symptoms of hypoglycaemia: confusion, dizziness, sweating, trembling, rapid heartbeat, or difficulty concentrating. If you have a blood glucose meter, check your levels immediately. Hypoglycaemia requires prompt treatment with 15-20g of fast-acting carbohydrates (such as glucose tablets, fruit juice, or sugary drinks), followed by a longer-acting carbohydrate snack once symptoms improve.
-
Signs of dehydration: dark urine, infrequent urination, dry mouth, dizziness upon standing, or persistent thirst, particularly if you have had ongoing gastrointestinal symptoms.
-
Symptoms suggesting pancreatitis: severe, persistent abdominal pain (often radiating to the back), nausea, and vomiting. Acute pancreatitis is a rare but serious potential adverse effect of GLP-1 receptor agonists.
-
Cardiovascular symptoms: chest pain, shortness of breath, or palpitations alongside fatigue.
-
Visual changes: any new or worsening vision problems, as rapid improvement in blood glucose control can sometimes worsen diabetic retinopathy temporarily.
-
Neck symptoms: a lump in the neck, persistent hoarseness, or difficulty swallowing should be reported promptly.
Your healthcare provider can conduct appropriate investigations, which may include blood tests to assess glucose control (HbA1c), kidney function, liver function, thyroid function, full blood count (to exclude anaemia), and electrolyte levels. They can determine whether fatigue is related to Mounjaro, requires dose adjustment, or indicates another underlying condition requiring treatment. According to NICE guidance on diabetes management (NG28), regular monitoring and individualised treatment adjustments are essential components of safe, effective care.
Other Common Side Effects of Mounjaro
Beyond fatigue, Mounjaro is associated with several other side effects that patients should be aware of. Gastrointestinal symptoms are the most frequently reported adverse effects and include nausea, diarrhoea, vomiting, constipation, abdominal pain, and dyspepsia (indigestion). These effects are typically most pronounced when initiating treatment or increasing the dose, and often diminish over time as the body adjusts. Eating smaller, bland, lower-fat meals and staying well-hydrated can help manage these symptoms.
Decreased appetite is an intended pharmacological effect that contributes to weight loss but can be experienced as an adverse effect by some patients. Whilst reduced appetite supports weight management goals, it is important to ensure adequate nutritional intake to maintain overall health and prevent nutrient deficiencies.
Injection site reactions such as redness, itching, or mild discomfort at the injection site can occur. Rotating injection sites (abdomen, thigh, or upper arm) and ensuring proper injection technique can minimise these reactions. Your diabetes specialist nurse or practice nurse can provide guidance on optimal injection practices.
Hypoglycaemia (low blood sugar) is more likely to occur when Mounjaro is used in combination with other glucose-lowering medications, particularly insulin or sulfonylureas. Symptoms include sweating, trembling, hunger, confusion, and palpitations. Your healthcare team may adjust doses of concurrent medications to reduce this risk.
Important safety information: The MHRA SmPC notes that tirzepatide may reduce the exposure to oral contraceptives if taken shortly after a dose increase. If using oral contraceptives, consider using a barrier method for 4 weeks after each dose escalation. Mounjaro is not recommended during pregnancy or breastfeeding, and women of childbearing potential should use effective contraception. Patients with diabetic retinopathy should be monitored closely, as rapid improvements in glucose control have been associated with temporary worsening of retinopathy.
Rare but serious adverse effects include acute pancreatitis (severe abdominal pain), gallbladder problems (cholecystitis or cholelithiasis), and acute kidney injury (particularly in the context of dehydration). Animal studies have shown an increased incidence of thyroid C-cell tumours, though the relevance to humans is unknown. Patients should report any symptoms such as a neck mass, hoarseness, or difficulty swallowing.
The MHRA's Yellow Card scheme allows patients and healthcare professionals to report suspected side effects, contributing to ongoing medication safety monitoring. If you experience any concerning symptoms whilst taking Mounjaro, discuss them with your healthcare provider to ensure appropriate management and continued safe use of this medication.
Scientific References
Frequently Asked Questions
How common is fatigue with Mounjaro?
According to the MHRA-approved Summary of Product Characteristics, fatigue is a common side effect of Mounjaro, potentially affecting up to 1 in 10 people taking the medication.
Why does Mounjaro cause tiredness?
Fatigue with Mounjaro may result from reduced caloric intake due to decreased appetite, blood glucose fluctuations, gastrointestinal side effects causing dehydration, or the body's metabolic adaptation to weight loss and improved glycaemic control.
When should I contact my GP about fatigue whilst taking Mounjaro?
Contact your GP if fatigue is severe, persistent beyond initial weeks, or accompanied by symptoms of hypoglycaemia, dehydration, severe abdominal pain, chest pain, shortness of breath, or visual changes. These may require urgent medical assessment.
The health-related content published on this site is based on credible scientific sources and is periodically reviewed to ensure accuracy and relevance. Although we aim to reflect the most current medical knowledge, the material is meant for general education and awareness only.
The information on this site is not a substitute for professional medical advice. For any health concerns, please speak with a qualified medical professional. By using this information, you acknowledge responsibility for any decisions made and understand we are not liable for any consequences that may result.
Heading 1
Heading 2
Heading 3
Heading 4
Heading 5
Heading 6
Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut aliquip ex ea commodo consequat. Duis aute irure dolor in reprehenderit in voluptate velit esse cillum dolore eu fugiat nulla pariatur.
Block quote
Ordered list
- Item 1
- Item 2
- Item 3
Unordered list
- Item A
- Item B
- Item C
Bold text
Emphasis
Superscript
Subscript












