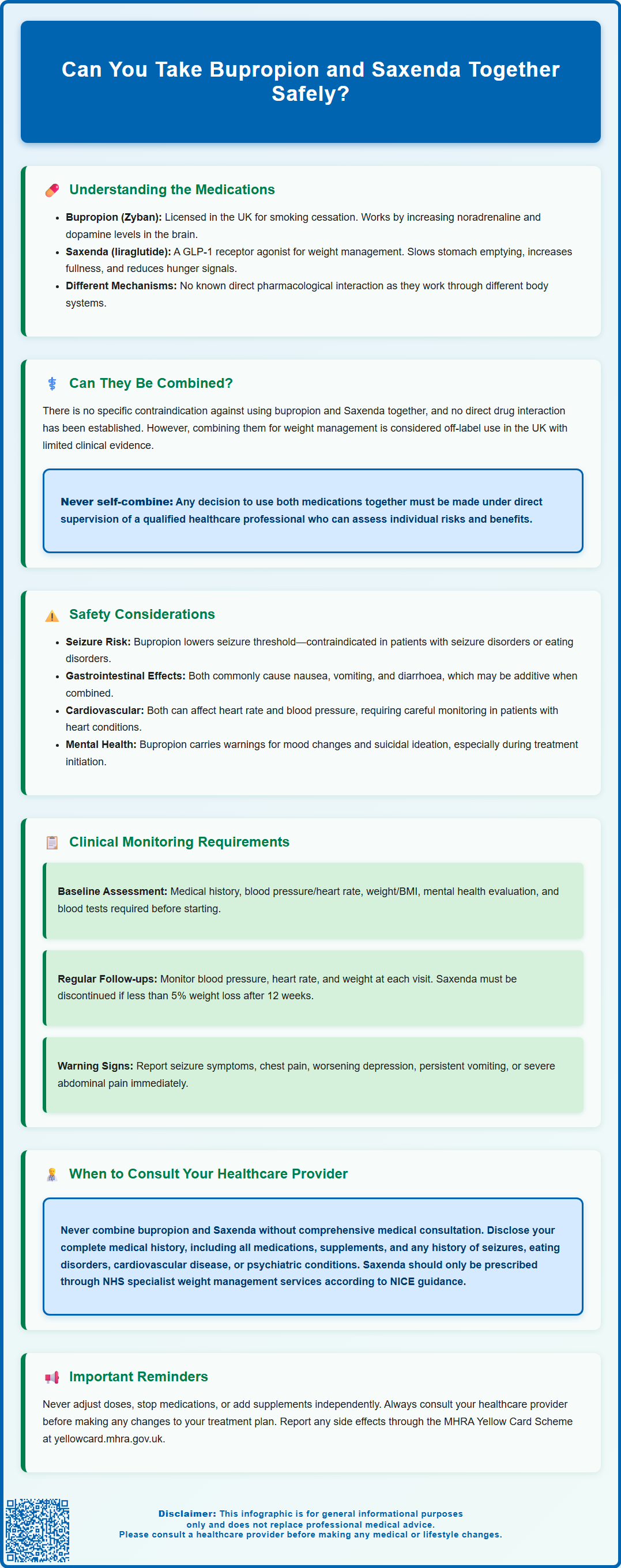
Can you take bupropion and Saxenda together? This question arises for patients who may require treatment for both smoking cessation and weight management. Bupropion (Zyban) is licensed in the UK for smoking cessation, whilst Saxenda (liraglutide) is a GLP-1 receptor agonist approved for weight management in adults with obesity or overweight with comorbidities. Whilst there is no specific contraindication against concurrent use, combining these medications requires careful medical assessment. This article examines the mechanisms of action, potential interactions, safety considerations, and the importance of specialist supervision when considering combination therapy with bupropion and Saxenda.
Summary: Bupropion and Saxenda can potentially be taken together as there is no specific contraindication, but combination therapy must only be undertaken under direct medical supervision with individualised risk assessment and monitoring.
- Bupropion is a noradrenaline-dopamine reuptake inhibitor licensed in the UK only for smoking cessation (as Zyban), whilst Saxenda (liraglutide) is a GLP-1 receptor agonist licensed for weight management.
- No direct pharmacological interaction exists between bupropion and liraglutide, but combining them for weight management constitutes off-label use with limited clinical evidence.
- Bupropion lowers seizure threshold and is contraindicated in patients with seizure disorders, eating disorders, or during abrupt alcohol or benzodiazepine withdrawal.
- Both medications may cause gastrointestinal effects, and liraglutide carries risks of pancreatitis and gallbladder disease requiring immediate medical attention if severe abdominal pain occurs.
- NICE guidance mandates that Saxenda should only be prescribed within specialist NHS weight management services and discontinued if 5% weight loss is not achieved after 12 weeks at maintenance dose.
- Patients require structured monitoring including blood pressure, heart rate, mental health status, and renal function, with immediate reporting of seizure warning signs or psychiatric changes through established healthcare channels.
Table of Contents
- Understanding Bupropion and Saxenda: Different Mechanisms of Action
- Can You Take Bupropion and Saxenda Together?
- Potential Drug Interactions and Safety Considerations
- Clinical Monitoring When Using Both Medications
- Consulting Your Healthcare Provider Before Combining Treatments
- Scientific References
- Frequently Asked Questions
Understanding Bupropion and Saxenda: Different Mechanisms of Action
Bupropion (marketed as Zyban in the UK) and Saxenda (liraglutide) are two distinct medications that work through different mechanisms within the body. Understanding how each functions is essential when considering their combined use.
Bupropion is licensed in the UK only for smoking cessation (as Zyban). It works by inhibiting the reuptake of noradrenaline and dopamine, thereby increasing the availability of these chemicals in neural pathways. It does not belong to the selective serotonin reuptake inhibitor (SSRI) class, which distinguishes it from many commonly prescribed antidepressants. While bupropion may occasionally be prescribed by specialists for depression in the UK, this would be an unlicensed (off-label) use. Bupropion's dopaminergic activity may contribute to modest weight loss in some patients, though this is not its primary indication.
Saxenda is a glucagon-like peptide-1 (GLP-1) receptor agonist specifically licensed for weight management in adults with obesity (BMI ≥30 kg/m²) or overweight (BMI ≥27 kg/m²) with weight-related comorbidities. For people of South Asian, Chinese, other Asian, Middle Eastern, Black African, or African-Caribbean family backgrounds, lower BMI thresholds may apply. Liraglutide mimics the action of the naturally occurring hormone GLP-1, which regulates appetite and food intake. It works by slowing gastric emptying, increasing feelings of satiety, and reducing hunger signals both in the gut and centrally in the brain. Saxenda is administered as a once-daily subcutaneous injection and is intended for use alongside a reduced-calorie diet and increased physical activity.
According to NICE Technology Appraisal 664, liraglutide should only be prescribed within specialist weight management services. Treatment should be stopped if at least 5% weight loss has not been achieved after 12 weeks on the maintenance dose, and treatment should not continue beyond 2 years.
While these medications target primarily different physiological systems, there is no known direct pharmacological interaction between them, which is an important consideration when evaluating potential combination therapy.
Can You Take Bupropion and Saxenda Together?
The question of whether bupropion and Saxenda can be taken concurrently does not have a straightforward answer. According to the Summary of Product Characteristics (SmPCs) for both medications, there is no specific contraindication against using bupropion and liraglutide together, and no direct pharmacological interaction between these two agents has been definitively established in clinical literature. However, this does not automatically mean the combination is appropriate for every patient.
It's important to understand that combining bupropion and liraglutide for weight management would be considered off-label use in the UK, and there is limited clinical evidence supporting this specific combination. While bupropion itself may contribute to weight loss as a secondary effect, which could theoretically complement the weight management goals of Saxenda, this potential benefit remains largely theoretical without robust UK-based clinical evidence.
Several factors influence whether concurrent use is suitable. Firstly, both medications may be prescribed for different primary indications—bupropion for smoking cessation, and liraglutide specifically for weight management. In clinical practice, some patients may legitimately require treatment for both smoking cessation and obesity, making combination therapy a potential consideration. The decision must be individualised based on the patient's complete medical history, current medications, and specific health needs.
Crucially, the safety and appropriateness of combining these medications should never be determined by patients independently. Self-directed polypharmacy carries significant risks, including unrecognised drug interactions, compounded adverse effects, and inadequate monitoring of treatment response. Any decision to use bupropion and Saxenda together must be made under the direct supervision of a qualified healthcare professional who can assess the individual risk-benefit profile and provide appropriate clinical oversight throughout treatment.
Potential Drug Interactions and Safety Considerations
While there is no direct pharmacokinetic interaction documented between bupropion and liraglutide, several important safety considerations warrant careful attention when these medications are used concurrently.
Seizure risk represents a significant concern with bupropion. The medication lowers the seizure threshold in a dose-dependent manner. Bupropion is contraindicated in patients with a current or previous seizure disorder, bulimia or anorexia nervosa, brain tumours, and during abrupt withdrawal from alcohol or benzodiazepines. It should not be used with monoamine oxidase inhibitors (MAOIs) or within 14 days of stopping an MAOI. Other medications that lower seizure threshold (including antipsychotics, tramadol, and some antimalarials) may increase risk when combined with bupropion. The maximum recommended dose should never be exceeded, and patients should be counselled about seizure warning signs.
Gastrointestinal effects are common with Saxenda, particularly during dose titration. Nausea, vomiting, diarrhoea, and constipation affect a substantial proportion of patients starting liraglutide. Bupropion can also cause gastrointestinal disturbances, including nausea and dry mouth. When used together, these effects may be additive, potentially affecting treatment adherence and nutritional intake. Liraglutide may delay gastric emptying, which could theoretically affect the absorption of oral medications, though this is rarely clinically significant for most drugs.
Pancreatitis risk with liraglutide requires attention. Treatment should be discontinued immediately if pancreatitis is suspected (persistent, severe abdominal pain, sometimes radiating to the back, with or without vomiting). Patients with a history of pancreatitis should use liraglutide with caution. Liraglutide is also associated with increased risk of gallbladder disease, including gallstones and cholecystitis.
Cardiovascular considerations should be evaluated. Bupropion may cause dose-related increases in blood pressure and heart rate in some patients. Saxenda carries warnings regarding increased heart rate, and cardiovascular effects have been observed in clinical trials. Patients with pre-existing cardiovascular disease, uncontrolled hypertension, or significant cardiac risk factors require particularly careful assessment.
Hypoglycaemia risk with liraglutide is primarily a concern when used alongside insulin or sulfonylureas in patients with diabetes.
Pregnancy and breastfeeding: Liraglutide is not recommended during pregnancy or breastfeeding. Women of childbearing potential should use contraception while on treatment.
Psychiatric effects merit close attention. Bupropion carries warnings about neuropsychiatric adverse reactions, including mood changes, agitation, and, rarely, suicidal ideation, particularly during treatment initiation or dose changes. Any changes in mood, behaviour, or emergence of suicidal thoughts require immediate medical attention.
Suspected adverse reactions to either medication should be reported through the MHRA Yellow Card Scheme (yellowcard.mhra.gov.uk).
Clinical Monitoring When Using Both Medications
Patients prescribed both bupropion and Saxenda concurrently require structured clinical monitoring to ensure treatment safety and effectiveness. A comprehensive monitoring plan should be established at treatment initiation and maintained throughout therapy.
Baseline assessment before starting combination therapy should include a thorough medical history, current medication review (including over-the-counter preparations and supplements), blood pressure and heart rate measurement, weight and BMI documentation (in kg/m²), and assessment of mental health status. Baseline blood tests may include renal function (particularly important for liraglutide), liver function, fasting glucose, and HbA1c if diabetes or prediabetes is present. A cardiovascular risk assessment should be performed, especially in patients over 40 or with existing risk factors.
Regular follow-up appointments are essential during the initial months of treatment. Blood pressure and heart rate should be monitored at each visit, particularly during dose titration phases. Weight should be tracked to assess Saxenda's effectiveness—NICE guidance (TA664) mandates that liraglutide should be discontinued if patients have not lost at least 5% of initial body weight after 12 weeks at the maintenance dose. Treatment should not continue beyond 2 years. In the NHS, liraglutide for weight management should only be prescribed within specialist weight management services.
Mental health status requires ongoing evaluation, with particular attention to mood changes, anxiety levels, sleep patterns, and any emergence of suicidal ideation. Renal function should be monitored, particularly if severe gastrointestinal symptoms occur, as these may lead to dehydration and acute kidney injury.
Patient self-monitoring plays a vital role in treatment safety. Patients should be educated to recognise and report warning signs promptly, including:
-
Seizure warning signs: unusual sensations, confusion, loss of consciousness, or convulsive movements
-
Cardiovascular symptoms: chest pain, palpitations, severe dizziness, or shortness of breath
-
Psychiatric changes: worsening depression, anxiety, agitation, panic attacks, or thoughts of self-harm
-
Severe gastrointestinal symptoms: persistent vomiting, severe abdominal pain (which may indicate pancreatitis), or signs of dehydration
-
Hypoglycaemia symptoms (if diabetic): tremor, sweating, confusion, or rapid heartbeat
Medication adherence and technique should be reviewed regularly. For Saxenda, proper injection technique, site rotation, and correct storage of the pen device require verification. For bupropion, adherence to prescribed dosing schedules and avoidance of dose doubling if a dose is missed should be reinforced.
Women of childbearing potential should be advised about contraception while using liraglutide. If pregnancy occurs, liraglutide should be discontinued immediately and medical advice sought.
Consulting Your Healthcare Provider Before Combining Treatments
The decision to use bupropion and Saxenda together should never be made without comprehensive medical consultation. Self-directed combination of prescription medications is potentially dangerous and may result in serious adverse outcomes. Purchasing prescription medicines online without a valid prescription is both risky and potentially illegal.
Before initiating combination therapy, patients should schedule a thorough consultation with their GP or specialist. This appointment should include full disclosure of all current medications (prescription, over-the-counter, herbal, and supplements), complete medical history including any history of seizures, eating disorders, cardiovascular disease, psychiatric conditions, or substance use, and clear discussion of treatment goals and expectations for both medications. Patients should feel empowered to ask questions about why both medications are being considered, what benefits are anticipated, what risks are involved, and what alternatives might be available.
Specialist input is appropriate in most circumstances. In the NHS, Saxenda should only be prescribed within specialist weight management services according to NICE guidance. Your GP can refer you to these services if you meet the eligibility criteria. Patients with complex mental health needs may benefit from psychiatric consultation to optimise treatment. Those pursuing weight management may be referred to specialist weight management services, as recommended by NICE for patients considering pharmacological interventions for obesity. Endocrinology input may be valuable for patients with diabetes or metabolic complications.
Ongoing communication with healthcare providers is essential throughout treatment. Patients should never adjust doses independently, discontinue either medication without medical guidance, or add additional medications or supplements without informing their prescriber. Do not stop or change doses without medical advice; your prescriber will advise if tapering is needed when stopping medication. Any new symptoms, concerns about side effects, or questions about treatment should prompt contact with the healthcare team.
If you are currently taking one of these medications and your healthcare provider suggests adding the other, ensure you understand the rationale, receive clear instructions about dosing and monitoring, know what side effects to watch for and when to seek help, and have a follow-up plan established. Similarly, if you are considering requesting one of these medications while already taking the other, prepare for your appointment by documenting your current symptoms, treatment goals, and any concerns.
Ultimately, the safe and effective use of bupropion and Saxenda in combination requires individualised medical assessment, careful monitoring, and collaborative decision-making between patient and healthcare provider. This partnership approach optimises treatment outcomes whilst minimising potential risks.
If you experience any suspected side effects from either medication, report them through the MHRA Yellow Card Scheme at yellowcard.mhra.gov.uk.
Scientific References
- Saxenda 6 mg/mL solution for injection in pre-filled pen - Summary of Product Characteristics.
- Zyban 150 mg prolonged release tablets - Summary of Product Characteristics.
- Liraglutide for managing overweight and obesity - Technology appraisal guidance TA664.
- Obesity - Treatment.
- Saxenda - European Public Assessment Report.
- British National Formulary - Interactions A to Z.
Frequently Asked Questions
Is it safe to combine bupropion and Saxenda for weight loss?
Whilst there is no specific contraindication against using bupropion and Saxenda together, combining them for weight management is off-label use in the UK with limited clinical evidence. Any combination therapy must be prescribed and monitored by a qualified healthcare professional who can assess individual risks and benefits.
What are the main risks of taking bupropion and Saxenda together?
Key risks include bupropion's seizure threshold lowering effect, additive gastrointestinal side effects from both medications, cardiovascular effects including increased heart rate and blood pressure, and potential psychiatric symptoms. Saxenda also carries risks of pancreatitis and gallbladder disease requiring immediate medical attention.
Do I need specialist supervision to take bupropion and Saxenda together?
Yes, specialist supervision is essential. NICE guidance requires that Saxenda be prescribed only within specialist NHS weight management services, and combining it with bupropion requires individualised medical assessment, structured monitoring of blood pressure, heart rate, mental health status, and regular follow-up appointments to ensure treatment safety.
The health-related content published on this site is based on credible scientific sources and is periodically reviewed to ensure accuracy and relevance. Although we aim to reflect the most current medical knowledge, the material is meant for general education and awareness only.
The information on this site is not a substitute for professional medical advice. For any health concerns, please speak with a qualified medical professional. By using this information, you acknowledge responsibility for any decisions made and understand we are not liable for any consequences that may result.
Heading 1
Heading 2
Heading 3
Heading 4
Heading 5
Heading 6
Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut aliquip ex ea commodo consequat. Duis aute irure dolor in reprehenderit in voluptate velit esse cillum dolore eu fugiat nulla pariatur.
Block quote
Ordered list
- Item 1
- Item 2
- Item 3
Unordered list
- Item A
- Item B
- Item C
Bold text
Emphasis
Superscript
Subscript










