Mounjaro (tirzepatide) is a once-weekly injectable medication licensed in the UK for type 2 diabetes and weight management. The pre-filled pens use a colour-coded system to help identify different dosage strengths—2.5 mg, 5 mg, 7.5 mg, 10 mg, 12.5 mg, and 15 mg. Whilst pen colours provide a visual reference, patients must always verify the strength in milligrams printed on the carton and pen label before each injection. Understanding this identification system supports safe medication administration, particularly during dose escalation phases. This article explains the colour-coding system, how to identify your correct dose, and essential safety considerations when using Mounjaro pens.
Summary: Mounjaro pens use a colour-coded system to identify six dosage strengths (2.5–15 mg), but patients must always verify the exact milligram strength printed on the carton and pen label before injection.
- Tirzepatide is a GLP-1/GIP receptor agonist licensed in the UK for type 2 diabetes and weight management as an adjunct to lifestyle modification.
- Six dosage strengths are available (2.5 mg, 5 mg, 7.5 mg, 10 mg, 12.5 mg, 15 mg), each with distinct pen cap and label colours.
- Treatment typically starts at 2.5 mg weekly, with dose escalation every four weeks based on glycaemic response and tolerability to minimise gastrointestinal adverse effects.
- Patients should verify milligram strength on labels before each injection, check solution clarity, and store pens refrigerated (2–8°C) in original packaging.
- Contact your GP urgently if you inject the wrong dose, experience severe abdominal pain, persistent vomiting, or signs of allergic reaction or hypoglycaemia.
Table of Contents
Understanding Mounjaro Pen Colours and Dosage Identification
Mounjaro (tirzepatide) is a once-weekly injectable medication licensed in the UK for the treatment of type 2 diabetes mellitus and, more recently, for weight management in adults with obesity or overweight with at least one weight-related comorbidity, as an adjunct to reduced-calorie diet and increased physical activity. The medication is supplied in pre-filled, single-dose pens that utilise a colour-coded system to help patients and healthcare professionals identify different dosage strengths. This visual identification system is a safety feature designed to reduce medication errors, but should never be relied upon as the sole identifier.
The colour-coding system employed by Mounjaro pens serves multiple purposes within clinical practice. Firstly, it provides a visual reference that helps patients confirm they are using the correct strength, particularly important during dose escalation phases when treatment is being titrated upwards. Secondly, it assists healthcare professionals during patient consultations and medication reviews. Finally, the distinct colours help prevent dispensing errors in pharmacy settings and support medication reconciliation processes.
Understanding the relationship between pen colour and dosage strength is one element of safe medication administration. Each Mounjaro pen is designed for single use only and contains a fixed dose of tirzepatide. However, it is essential to always verify the strength in milligrams (mg) printed on both the carton and pen label, rather than relying solely on colour. If there is any uncertainty about pen identification or dosage, patients should contact their GP, diabetes specialist nurse, or community pharmacist before proceeding with injection.
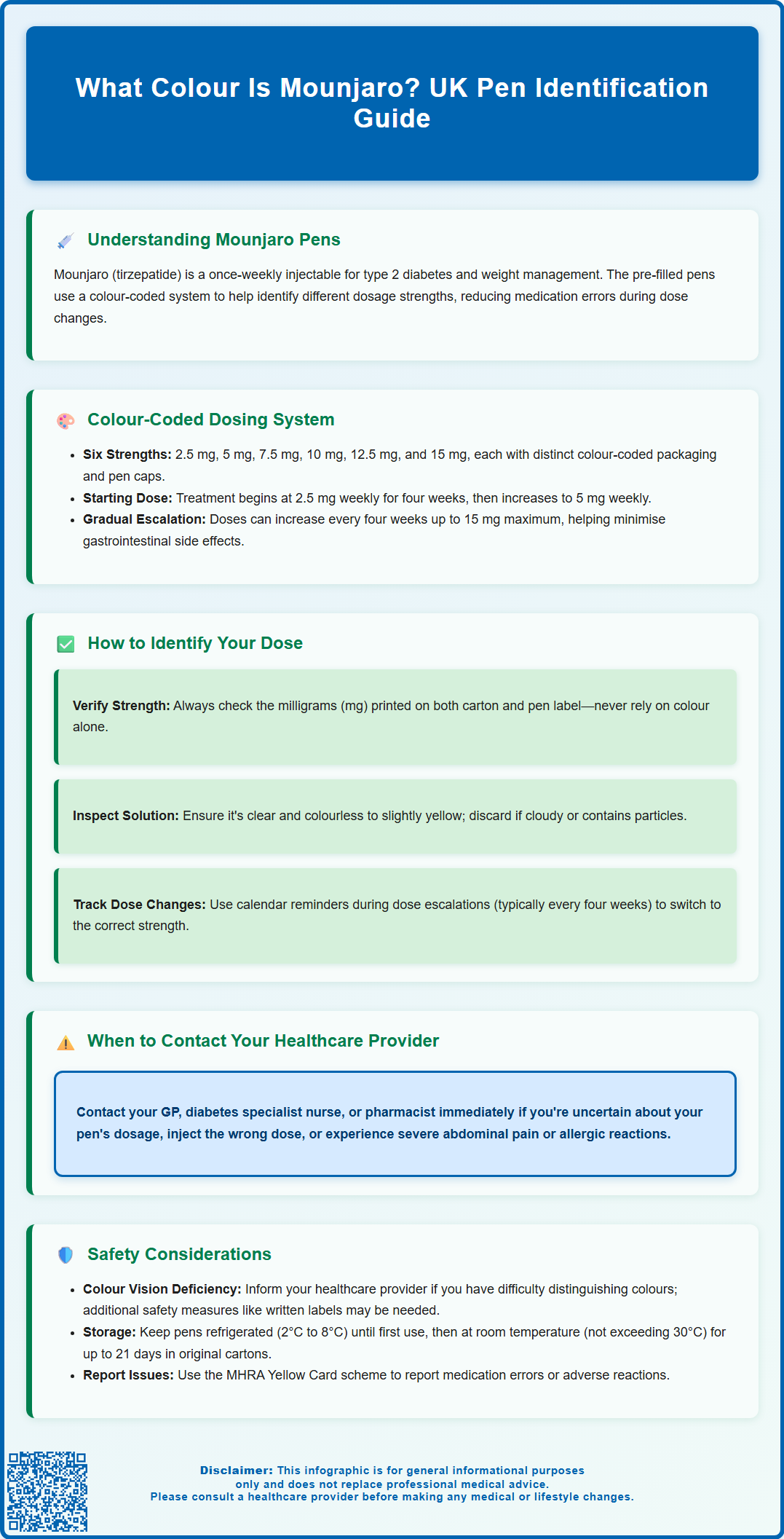
Mounjaro Colour-Coded Dosing System Explained
Mounjaro pens are available in six distinct dosage strengths in the UK: 2.5 mg, 5 mg, 7.5 mg, 10 mg, 12.5 mg, and 15 mg. Each strength has its own colour scheme on the packaging and pen to help with identification. However, as colour schemes can vary between markets and may change over time, patients should always primarily rely on the strength in milligrams clearly printed on the carton and pen label.
The graduated dosing system reflects the recommended dose escalation schedule outlined in the Summary of Product Characteristics (SmPC) approved by the Medicines and Healthcare products Regulatory Agency (MHRA). Treatment typically commences at 2.5 mg once weekly for four weeks, after which the dose is increased to 5 mg once weekly. Further dose increases can be made at four-week intervals based on individual glycaemic response and tolerability, up to a maximum of 15 mg once weekly. This stepwise titration approach helps minimise gastrointestinal adverse effects, which are the most commonly reported side effects with tirzepatide therapy. If adverse effects occur, dose escalation may be delayed by 2-4 weeks, and not all patients will require the maximum dose.
The colour-coding extends to both the pen cap and the label affixed to the pen body, providing visual confirmation of dosage strength. Each pen also displays the dose strength in milligrams clearly printed on the label. Healthcare professionals should educate patients about their specific pen during initiation and at each dose escalation, reinforcing the importance of verifying the strength in milligrams before each injection. Patients should store pens in their original packaging until use to maintain clear identification and protect the medication from light exposure, as per storage requirements.
How to Identify Your Mounjaro Dose by Pen Colour
Correctly identifying your Mounjaro pen before each injection is a fundamental aspect of safe medication practice. Begin by examining the pen whilst it remains in its original packaging. The outer carton will display the dosage strength prominently, and this should match your current prescription. Once removed from the carton, inspect the pen cap colour and cross-reference this with the printed label on the pen body, which states the exact dose in milligrams alongside the colour-coded design.
Patients should establish a routine verification process before each weekly injection. Key identification steps include:
-
Check the strength in milligrams (mg) printed on the carton and pen label
-
Note the pen cap colour as a secondary identifier
-
Verify the expiry date printed on the pen
-
Confirm the solution appears clear and colourless to slightly yellow (do not use if cloudy, discoloured or contains particles)
-
Ensure the pen has not been previously used (check the tamper-evident cap and injection indicator window)
It is particularly important to pay careful attention during dose escalation periods when you transition from one strength to another. Your prescriber or diabetes specialist nurse should provide clear instructions about when to change to the next strength, typically after four weeks at each dose level. Some patients find it helpful to mark their calendar or set phone reminders indicating when dose changes are scheduled, alongside noting the new pen strength they should be using.
If you receive a pen that does not match your prescription, or if you are uncertain about which strength you should be using, do not administer the injection. Contact your community pharmacist immediately for clarification, or speak with your GP surgery or diabetes clinic. Never attempt to adjust your dose independently or use a pen strength that differs from your prescription, as this could result in inadequate glycaemic control or increased risk of adverse effects.
Safety Considerations When Using Colour-Coded Mounjaro Pens
Whilst the colour-coded system enhances medication safety, several important considerations remain essential for optimal patient outcomes. Individuals with colour vision deficiency (colour blindness) may experience difficulty distinguishing between certain pen colours. If you have known colour vision impairment, inform your healthcare provider during treatment initiation. Additional safety measures can be implemented, such as clearly labelling pens with the written dose strength, involving a family member or carer in dose verification, or using pharmacy-dispensed labels with large-print dosage information.
Medication storage practices also impact safe pen identification. Mounjaro pens should be stored in a refrigerator (2°C to 8°C) until first use, and may then be kept at room temperature (not exceeding 30°C) for up to 21 days. Do not freeze. Always store pens in their original carton to protect from light and maintain clear identification. Never remove the pen cap until immediately before injection, as this protects the needle and prevents contamination. If you store multiple pen strengths simultaneously (for example, during a transition period), keep them in separate, clearly labelled containers to prevent selection errors.
Contact your GP or diabetes specialist nurse urgently if:
-
You accidentally inject the wrong dose strength
-
You experience severe or persistent gastrointestinal symptoms (nausea, vomiting, diarrhoea)
-
You develop severe, persistent abdominal pain, possibly radiating to the back (potential sign of pancreatitis)
-
You notice signs of gallbladder problems (pain in upper abdomen, fever, yellowing of skin/eyes)
-
You experience symptoms of a severe allergic reaction (rash, swelling, breathing difficulties)
-
You develop symptoms of hypoglycaemia, particularly if taking insulin or sulfonylureas (these medications may need dose adjustment)
-
You notice any changes in the medication's appearance
For severe allergic reactions or if you feel very unwell, contact NHS 111 or 999 as appropriate. The MHRA Yellow Card scheme (yellowcard.mhra.gov.uk) allows patients and healthcare professionals to report suspected adverse drug reactions or medication errors. Always attend scheduled follow-up appointments for monitoring of glycaemic control, weight changes, and assessment of treatment tolerability, ensuring your Mounjaro therapy remains both safe and effective.
Scientific References
Frequently Asked Questions
Can I rely solely on pen colour to identify my Mounjaro dose?
No, you must always verify the exact milligram strength printed on both the carton and pen label before injection. Colour-coding is a secondary visual aid only, and individuals with colour vision deficiency may have difficulty distinguishing between pen colours.
How often does my Mounjaro dose increase?
Mounjaro typically starts at 2.5 mg weekly for four weeks, then increases to 5 mg weekly. Further dose escalations occur at four-week intervals based on your glycaemic response and tolerability, up to a maximum of 15 mg weekly.
What should I do if I receive the wrong strength Mounjaro pen?
Do not administer the injection. Contact your community pharmacist immediately for clarification, or speak with your GP surgery or diabetes clinic to ensure you receive the correct prescribed strength before proceeding.
The health-related content published on this site is based on credible scientific sources and is periodically reviewed to ensure accuracy and relevance. Although we aim to reflect the most current medical knowledge, the material is meant for general education and awareness only.
The information on this site is not a substitute for professional medical advice. For any health concerns, please speak with a qualified medical professional. By using this information, you acknowledge responsibility for any decisions made and understand we are not liable for any consequences that may result.
Heading 1
Heading 2
Heading 3
Heading 4
Heading 5
Heading 6
Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut aliquip ex ea commodo consequat. Duis aute irure dolor in reprehenderit in voluptate velit esse cillum dolore eu fugiat nulla pariatur.
Block quote
Ordered list
- Item 1
- Item 2
- Item 3
Unordered list
- Item A
- Item B
- Item C
Bold text
Emphasis
Superscript
Subscript










