Wegovy (semaglutide 2.4 mg) is a GLP-1 receptor agonist licensed in the UK for weight management in adults with obesity or overweight with weight-related comorbidities. Some patients wonder whether Wegovy yeast infection concerns are valid. Yeast infections are not listed as a recognised adverse effect in the MHRA-approved Summary of Product Characteristics, and there is no established direct pharmacological mechanism linking semaglutide to Candida overgrowth. However, many patients prescribed Wegovy have underlying conditions such as type 2 diabetes, which independently increase yeast infection risk. This article examines the evidence, risk factors, and practical management strategies.
Summary: Wegovy (semaglutide) is not recognised as a direct cause of yeast infections according to UK regulatory authorities, though underlying conditions like diabetes in patients using Wegovy may independently increase infection risk.
- Wegovy is a GLP-1 receptor agonist licensed for weight management, not listed by the MHRA as causing yeast infections.
- Semaglutide works by enhancing insulin secretion, suppressing glucagon, slowing gastric emptying, and reducing appetite through central pathways.
- Many Wegovy patients have pre-existing diabetes or insulin resistance, which are independent risk factors for Candida overgrowth due to elevated blood glucose.
- Improved glycaemic control from Wegovy may actually reduce yeast infection risk over time in patients with diabetes.
- First-line treatments include topical clotrimazole pessaries or oral fluconazole 150 mg, with no significant drug interactions with semaglutide.
- Recurrent infections (four or more annually) require medical evaluation, vaginal swabs for culture, and assessment of underlying metabolic control.
Table of Contents
Can Wegovy Cause Yeast Infections?
Wegovy (semaglutide 2.4 mg) is a glucagon-like peptide-1 (GLP-1) receptor agonist licensed in the UK for weight management in adults with obesity or overweight with weight-related comorbidities. Yeast infections are not listed as a recognised adverse effect in the Summary of Product Characteristics (SmPC) approved by the Medicines and Healthcare products Regulatory Agency (MHRA), nor in the European Medicines Agency's European Public Assessment Report (EPAR) for Wegovy.
There is no established direct pharmacological mechanism by which semaglutide would cause yeast infections. The drug works primarily by mimicking the incretin hormone GLP-1, which enhances glucose-dependent insulin secretion, suppresses glucagon release, slows gastric emptying, and reduces appetite through central nervous system pathways. None of these actions directly affect the vaginal or skin microbiome in ways that would promote Candida overgrowth.
However, the metabolic conditions of patients using Wegovy may influence infection risk. Many patients prescribed Wegovy have pre-existing conditions such as type 2 diabetes or prediabetes, which are independent risk factors for recurrent yeast infections due to elevated blood glucose levels that favour Candida proliferation. In patients with diabetes, as Wegovy improves glycaemic control and promotes weight loss, the overall risk profile for yeast infections may actually improve over time. Any apparent association between Wegovy and yeast infections is more likely related to underlying metabolic conditions rather than a direct drug effect.
If you suspect Wegovy may be associated with any adverse effect, including yeast infections, you can report this through the MHRA Yellow Card scheme. Patients concerned about recurrent infections should discuss their individual risk factors with their healthcare provider.
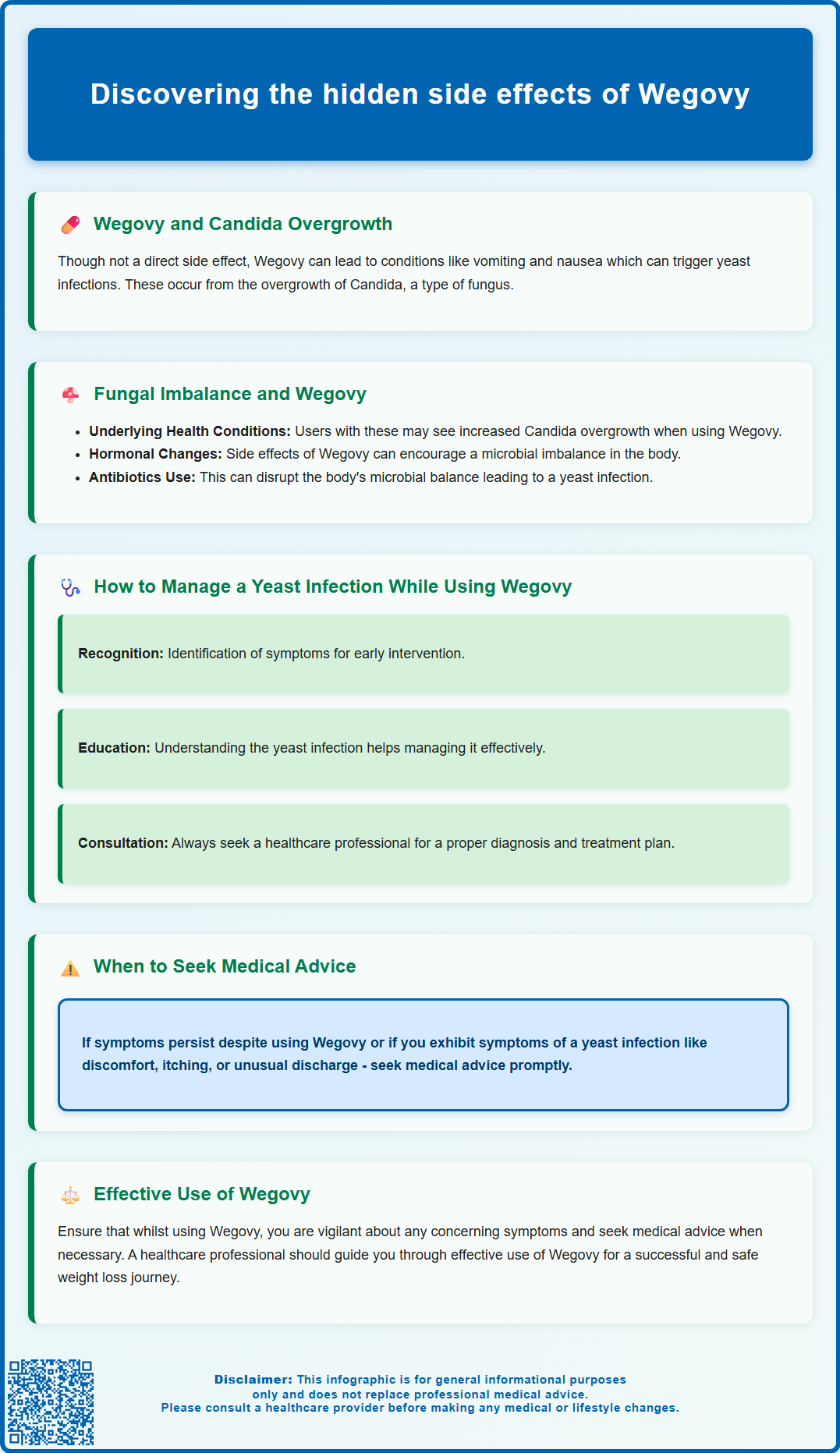
Why Yeast Infections May Occur During Wegovy Treatment
Several established risk factors may explain why some individuals experience yeast infections whilst taking Wegovy, even though there is no official causal link. Understanding these contributing elements helps patients and clinicians identify modifiable risk factors and implement appropriate preventive strategies.
Pre-existing diabetes or insulin resistance represents the most significant risk factor. Many patients prescribed Wegovy have type 2 diabetes or metabolic syndrome, conditions associated with chronically elevated blood glucose. Candida albicans, the fungus responsible for most yeast infections, thrives in glucose-rich environments. Higher glucose concentrations in vaginal secretions, urine, and skin folds create ideal conditions for fungal overgrowth. Whilst Wegovy improves glycaemic control in patients with diabetes, patients may still experience fluctuating glucose levels during the initial treatment period.
Other well-established risk factors for vulvovaginal candidiasis, according to the British Association for Sexual Health and HIV (BASHH) guidelines, include recent antibiotic use, pregnancy, immunosuppression (including high-dose corticosteroids), and certain contraceptives. The NHS also identifies tight-fitting synthetic clothing, damaged skin, and certain underlying health conditions as contributing factors.
Recognising Symptoms of a Yeast Infection
Prompt recognition of yeast infection symptoms enables timely treatment and prevents progression to more troublesome infections. Vulvovaginal candidiasis, the most common form of yeast infection in women, presents with characteristic features that distinguish it from other genital conditions.
The hallmark symptom is intense vulval itching (pruritus), which may be persistent and significantly affect quality of life. This itching typically worsens at night or after bathing and is often accompanied by a burning sensation, particularly during urination or sexual intercourse. Women frequently describe vulval soreness, redness, and swelling of the labia and surrounding tissues. The vaginal discharge associated with candidiasis is classically described as thick, white, and cottage cheese-like in appearance, without an offensive odour. However, discharge characteristics can vary, and some women experience minimal discharge despite significant symptoms.
Physical examination may reveal erythema and oedema of the vulva and vagina, sometimes with visible white plaques adherent to the vaginal walls. Fissuring or cracking of the vulval skin can occur in severe cases, causing additional discomfort.
It is important to note that not all vaginal symptoms indicate yeast infection. Bacterial vaginosis typically presents with a thin, grey-white discharge with a fishy odour, while trichomoniasis often causes a yellow-green frothy discharge. Sexually transmitted infections may present with additional symptoms such as pelvic pain or unusual bleeding. Dermatological conditions and irritant reactions can also produce similar presentations.
Men can also develop genital yeast infections (candidal balanitis), though less commonly. Symptoms include redness, itching, and irritation of the glans penis and foreskin, sometimes with a white discharge beneath the foreskin. Oral thrush, characterised by white patches on the tongue and inner cheeks, represents another manifestation of Candida infection, though this is uncommon in healthy adults; there is no evidence that Wegovy increases this risk.
Managing and Preventing Yeast Infections While Taking Wegovy
Effective management of yeast infections during Wegovy treatment involves both appropriate treatment of active infections and implementation of preventive strategies to reduce recurrence risk. Most uncomplicated yeast infections respond well to antifungal therapy available without prescription from UK pharmacies.
For acute vulvovaginal candidiasis, topical imidazole antifungals such as clotrimazole pessaries (500 mg single dose or 200 mg for three nights) or miconazole cream represent first-line treatment options according to NICE Clinical Knowledge Summaries. Oral fluconazole 150 mg as a single dose offers a convenient alternative and is equally effective for uncomplicated infections in non-pregnant women. However, oral fluconazole should be avoided during pregnancy; intravaginal clotrimazole is the preferred option for pregnant women. Patients should check with a pharmacist about potential drug interactions, particularly if taking anticoagulants like warfarin, as fluconazole can increase their effect.
These treatments are generally safe to use alongside Wegovy, as there are no significant drug interactions between semaglutide and common antifungal medications. Symptoms typically improve within a few days, though complete resolution may take up to a week. If symptoms persist beyond seven days despite treatment, medical review is warranted.
Prevention strategies focus on modifying risk factors and maintaining optimal vulvovaginal health. Glycaemic control remains paramount—patients with diabetes should monitor blood glucose levels regularly and work with their healthcare team to optimise diabetic management alongside Wegovy treatment. NICE guidance (NG28) recommends individualised HbA1c targets, typically around 48 mmol/mol (6.5%) for most adults with type 2 diabetes, or up to 53 mmol/mol (7.0%) for those at risk of hypoglycaemia or with other individual factors.
Practical hygiene measures include wearing breathable cotton underwear, avoiding tight-fitting synthetic clothing, and changing out of damp clothing promptly after exercise or swimming. Gentle washing with water or unperfumed soap is preferable to harsh cleansers, douches, or feminine hygiene products that disrupt the natural vaginal flora. Probiotics are not routinely recommended for prevention of vulvovaginal candidiasis due to limited evidence.
For patients experiencing recurrent infections (four or more episodes annually), BASHH guidelines recommend vaginal swabs for culture and Candida species identification, as non-albicans species may require different treatment approaches. Longer courses of antifungal therapy or suppressive maintenance regimens (such as fluconazole 150 mg weekly for 6 months) may be appropriate under specialist guidance from a GP or sexual health service.
When to Seek Medical Advice
Whilst many yeast infections can be managed with over-the-counter treatments, certain circumstances warrant professional medical assessment to ensure accurate diagnosis, exclude alternative conditions, and identify underlying factors requiring specific intervention.
Patients should contact their GP or sexual health clinic if experiencing their first suspected yeast infection, as self-diagnosis can be unreliable and other conditions may present similarly. Bacterial vaginosis, trichomoniasis, genital herpes, and contact dermatitis can mimic candidiasis symptoms, and appropriate diagnosis ensures correct treatment. Women who are pregnant or breastfeeding should seek medical advice before using antifungal treatments, as oral fluconazole is contraindicated in pregnancy and some formulations require caution during breastfeeding.
Recurrent yeast infections—defined as four or more episodes within twelve months—require medical evaluation to investigate potential underlying causes. According to BASHH guidelines, vaginal swabs for culture and Candida species identification should be performed to detect non-albicans species or azole resistance. Poorly controlled diabetes, immunosuppression, antibiotic use, or other predisposing factors may need addressing. Blood tests to check HbA1c, fasting glucose, or immune function may be appropriate.
Patients should seek urgent medical attention if they develop:
-
Severe symptoms including extensive vulval swelling or ulceration
-
Systemic features such as fever
-
Pregnancy with severe symptoms
-
Immunocompromised state with infection
-
Unusual vaginal discharge with offensive odour, particularly if accompanied by pelvic pain, which suggests possible pelvic inflammatory disease requiring prompt antibiotic therapy
-
Any vaginal bleeding outside normal menstruation
It is important to note that over-the-counter fluconazole has specific licensing restrictions and may not be available without prescription for all patients; a pharmacist can advise on eligibility.
Patients should inform healthcare providers about Wegovy treatment and any other medications, as this context helps clinicians assess potential contributing factors and drug interactions. If you suspect Wegovy may be associated with any adverse effect, report this through the MHRA Yellow Card scheme. Patients should not discontinue Wegovy due to yeast infections without discussing this with the prescribing clinician, as the metabolic benefits of continued treatment typically outweigh the inconvenience of manageable infections.
Scientific References
- Wegovy 0.25 mg, FlexTouch solution for injection in pre-filled pen - Summary of Product Characteristics (SmPC).
- Wegovy: EPAR - Medicine Overview.
- The Yellow Card scheme: guidance for healthcare professionals.
- British Association for Sexual Health and HIV national guideline for the management of vulvovaginal candidiasis (2019).
- Vaginal and vulval conditions: Treatment summaries.
- Thrush in men and women.
Frequently Asked Questions
Does Wegovy directly cause yeast infections?
No, yeast infections are not listed as a recognised adverse effect of Wegovy by the MHRA, and there is no established pharmacological mechanism linking semaglutide to Candida overgrowth. Any association is more likely related to underlying conditions such as diabetes.
Can I use over-the-counter antifungal treatments while taking Wegovy?
Yes, topical clotrimazole pessaries and oral fluconazole are generally safe to use alongside Wegovy, as there are no significant drug interactions between semaglutide and common antifungal medications. Consult a pharmacist if taking other medications like warfarin.
When should I see a doctor for a yeast infection while on Wegovy?
Seek medical advice if experiencing your first suspected infection, if pregnant or breastfeeding, if symptoms persist beyond seven days despite treatment, or if you have recurrent infections (four or more episodes within twelve months).
The health-related content published on this site is based on credible scientific sources and is periodically reviewed to ensure accuracy and relevance. Although we aim to reflect the most current medical knowledge, the material is meant for general education and awareness only.
The information on this site is not a substitute for professional medical advice. For any health concerns, please speak with a qualified medical professional. By using this information, you acknowledge responsibility for any decisions made and understand we are not liable for any consequences that may result.
Heading 1
Heading 2
Heading 3
Heading 4
Heading 5
Heading 6
Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut aliquip ex ea commodo consequat. Duis aute irure dolor in reprehenderit in voluptate velit esse cillum dolore eu fugiat nulla pariatur.
Block quote
Ordered list
- Item 1
- Item 2
- Item 3
Unordered list
- Item A
- Item B
- Item C
Bold text
Emphasis
Superscript
Subscript












