Mounjaro (tirzepatide) is a dual GIP/GLP-1 receptor agonist licensed in the UK for type 2 diabetes and weight management in adults with obesity. Whilst not specifically approved for obstructive sleep apnoea (OSA), emerging clinical evidence suggests that the substantial weight loss achieved with Mounjaro may significantly improve OSA outcomes in patients whose condition is related to excess body weight. This article examines the clinical evidence, NHS guidance, and safety considerations for UK patients exploring Mounjaro's potential role in managing obesity-related sleep apnoea.
Summary: Mounjaro (tirzepatide) is not licensed for sleep apnoea but may improve obstructive sleep apnoea outcomes through significant weight loss when prescribed for type 2 diabetes or obesity management.
- Mounjaro is a dual GIP/GLP-1 receptor agonist administered as a once-weekly subcutaneous injection, starting at 2.5 mg with gradual dose escalation.
- Clinical trials show tirzepatide reduces apnoea-hypopnoea index by approximately 27 events per hour alongside 18–20% body weight loss over 52 weeks.
- Obesity is the most important modifiable risk factor for obstructive sleep apnoea, with weight loss of 10–15% producing meaningful improvements in OSA severity.
- Patients using CPAP therapy should not discontinue treatment when starting Mounjaro without guidance from their sleep specialist or respiratory physician.
- Common side effects include nausea, diarrhoea, and reduced appetite; serious risks include pancreatitis, gallbladder disease, and hypoglycaemia when combined with certain diabetes medications.
- NHS access typically requires specialist initiation through tier 3 weight management services following NICE criteria and local commissioning arrangements.
Table of Contents
What Is Mounjaro and How Does It Work?
Mounjaro (tirzepatide) is a prescription medicine licensed in the UK for the treatment of type 2 diabetes mellitus and, more recently, for weight management in adults with obesity or overweight with weight-related health conditions. It belongs to a novel class of medications known as dual glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptor agonists. This dual mechanism distinguishes Mounjaro from other GLP-1 receptor agonists currently available.
The medication works by mimicking the action of two naturally occurring incretin hormones. GLP-1 stimulates insulin secretion when blood glucose levels are elevated, suppresses glucagon release, slows gastric emptying, and reduces appetite through central nervous system pathways. GIP complements these effects by enhancing insulin secretion and may also influence fat metabolism, though its precise contribution to weight loss is not fully understood.
Mounjaro is administered as a once-weekly subcutaneous injection, with doses typically starting at 2.5 mg for 4 weeks, then increasing to 5 mg. Further dose increases to 7.5 mg, 10 mg, 12.5 mg, or 15 mg may be made at intervals of at least 4 weeks, depending on individual response and tolerability. Patients receive the medication via pre-filled injection pens, which are designed for self-administration following appropriate training from healthcare professionals.
Whilst Mounjaro is not specifically licensed for the treatment of obstructive sleep apnoea (OSA), emerging clinical evidence suggests that the substantial weight loss achieved with this medication may offer therapeutic benefits for patients whose sleep apnoea is related to excess body weight. It is important to emphasise that Mounjaro should only be prescribed within its licensed indications for type 2 diabetes or weight management.
The Link Between Weight Loss and Sleep Apnoea
Obstructive sleep apnoea (OSA) is a common sleep disorder characterised by repeated episodes of partial or complete upper airway obstruction during sleep, leading to intermittent hypoxia, sleep fragmentation, and daytime symptoms such as excessive sleepiness. In the UK, it is estimated that approximately 1.5 million adults have OSA, though many cases remain undiagnosed. Obesity is the single most important modifiable risk factor for OSA, with studies indicating that a significant proportion of people with OSA are overweight or obese.
Excess adipose tissue, particularly around the neck and upper airway, contributes to airway narrowing and collapse during sleep. Visceral fat deposition also promotes systemic inflammation and metabolic dysfunction, which may further exacerbate OSA severity. Research from the Wisconsin Sleep Cohort Study has demonstrated that weight gain can significantly increase OSA risk, while weight reduction has been shown to improve OSA outcomes.
Clinical studies consistently demonstrate that intentional weight loss of 10–15% can lead to meaningful reductions in the apnoea-hypopnoea index (AHI), the primary measure of OSA severity. Many patients experience substantial improvement with sufficient weight loss, which may reduce the need for continuous positive airway pressure (CPAP) therapy, though complete resolution of OSA is less common. Weight loss also improves associated metabolic complications, including hypertension, type 2 diabetes, and cardiovascular risk—conditions frequently comorbid with OSA.
Patients with suspected OSA should consult their GP, who may refer them to a sleep service based on symptoms and risk factors. Those experiencing excessive daytime sleepiness should follow DVLA guidance regarding driving safety. For mild to moderate OSA, mandibular advancement devices are an alternative treatment option supported by NICE guidance. Given the impact of obesity on OSA pathophysiology, pharmacological interventions that produce clinically significant weight loss, such as Mounjaro, represent a potentially valuable approach for patients with obesity-related sleep apnoea, alongside appropriate OSA-specific therapies.
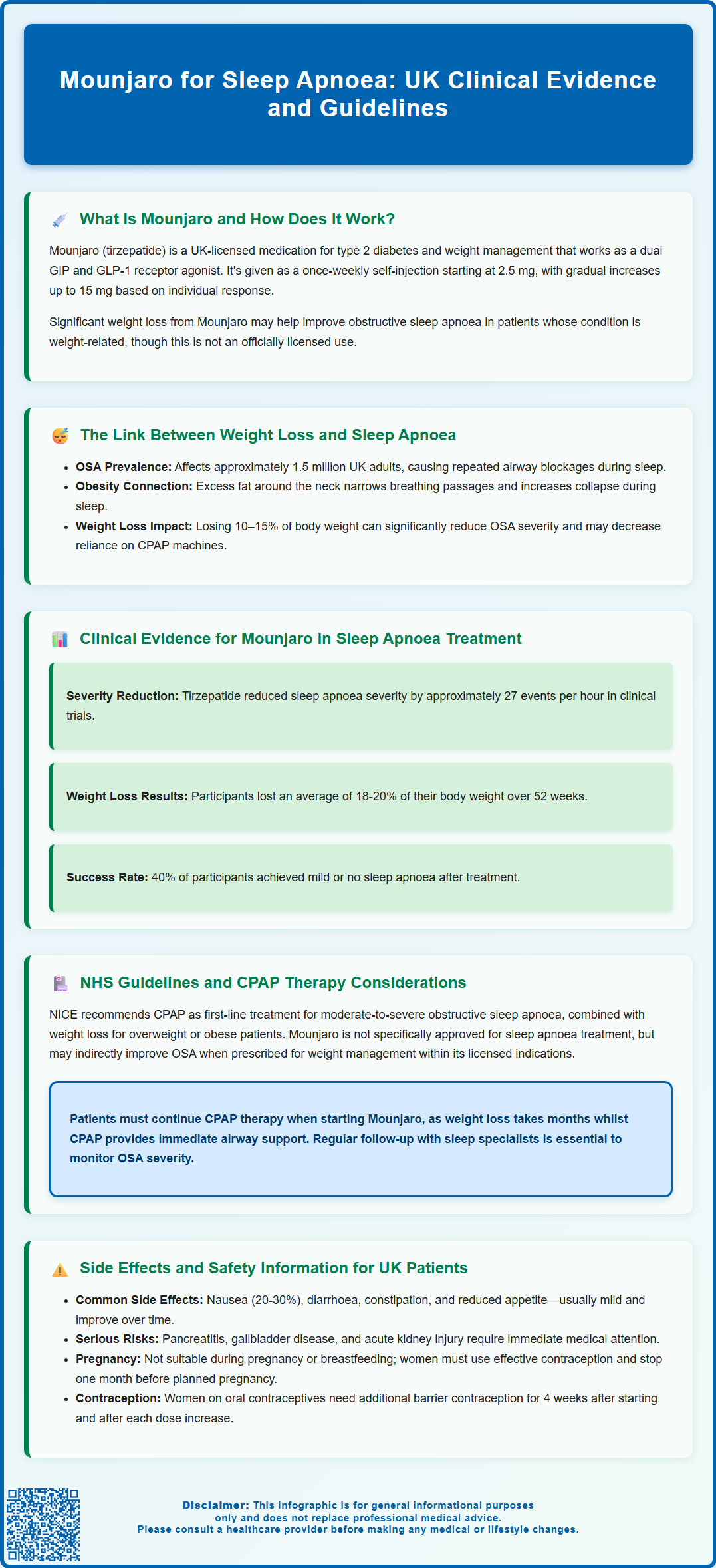
Clinical Evidence for Mounjaro in Sleep Apnoea Treatment
Recent clinical trial data have provided compelling evidence for the role of tirzepatide (Mounjaro) in improving obstructive sleep apnoea outcomes. The SURMOUNT-OSA trials, published in the New England Journal of Medicine in 2024, specifically investigated the efficacy of tirzepatide in adults with obesity and moderate-to-severe OSA. These consisted of two parallel randomised, double-blind, placebo-controlled studies: one in participants not using positive airway pressure (PAP) therapy and another in those using PAP therapy. Both enrolled participants with a body mass index (BMI) ≥30 kg/m² and an AHI of 15–65 events per hour.
In these landmark trials, participants receiving tirzepatide (titrated to 10 mg or 15 mg weekly) achieved an average reduction in AHI of approximately 27 events per hour compared to baseline in the non-PAP study, representing a significant improvement in OSA severity compared to placebo. Approximately 40% of participants in the tirzepatide group achieved an AHI below 15 events per hour—the threshold for moderate OSA—effectively moving them into the mild or no OSA category.
Weight loss was the primary driver of these improvements, with trial participants losing an average of 18–20% of their initial body weight over the 52-week study period. Secondary outcomes also showed improvements in patient-reported measures of sleep quality, daytime functioning, and cardiovascular risk markers including blood pressure.
Whilst these results are promising, it is important to emphasise that there is no official indication for Mounjaro specifically for sleep apnoea treatment in the UK at present. The medication is prescribed for type 2 diabetes or obesity management according to NICE guidance and local commissioning arrangements, and any benefits for OSA are considered secondary to weight reduction. Healthcare professionals considering tirzepatide for patients with comorbid OSA should ensure that prescribing aligns with current licensed indications and that patients continue appropriate OSA-specific therapies during treatment.
NHS Guidelines and CPAP Therapy Considerations
NICE guidance for obstructive sleep apnoea emphasises a multidisciplinary approach to management, with treatment decisions based on OSA severity, symptom burden, and individual patient factors. First-line therapy for moderate-to-severe OSA typically involves continuous positive airway pressure (CPAP), which provides pneumatic splinting of the upper airway during sleep. NICE also recommends lifestyle modifications, including weight loss, as an essential component of OSA management for all patients who are overweight or obese.
Currently, NICE does not specifically recommend GLP-1 receptor agonists or dual GIP/GLP-1 agonists like Mounjaro for the primary treatment of sleep apnoea. However, NICE technology appraisals support the use of tirzepatide for treating type 2 diabetes, and guidance on its use for weight management is being developed. For patients with both obesity and OSA, prescribing Mounjaro for weight management may indirectly benefit their sleep apnoea, provided the medication is used within its licensed indications.
Patients using CPAP therapy should not discontinue their treatment when starting Mounjaro without explicit guidance from their sleep specialist or respiratory physician. CPAP provides immediate therapeutic benefit by maintaining airway patency, whereas weight loss with tirzepatide occurs gradually over months. Regular follow-up with sleep services is essential to monitor OSA severity through repeat sleep studies (polysomnography or home sleep apnoea testing) and to adjust CPAP settings or consider therapy de-escalation as weight loss progresses.
NHS access to Mounjaro for weight management typically requires specialist initiation through tier 3 weight management services, following NICE criteria and local commissioning arrangements. Patients should discuss their eligibility with their GP or weight management team. Private prescribing is available but involves significant out-of-pocket costs. Regardless of access route, ongoing monitoring of both metabolic parameters and OSA symptoms is essential to optimise outcomes and ensure patient safety.
Side Effects and Safety Information for UK Patients
Like all medications, Mounjaro can cause side effects, and patients should be fully informed before starting treatment. The most commonly reported adverse effects are gastrointestinal in nature and include:
-
Nausea (affecting up to 20–30% of patients, particularly during dose escalation)
-
Diarrhoea and constipation
-
Vomiting and abdominal discomfort
-
Reduced appetite (which contributes to weight loss but may be troublesome for some)
These gastrointestinal symptoms are typically mild to moderate in severity and tend to improve over time as the body adjusts to the medication. Starting at a low dose and gradually increasing, as per the prescribing protocol, helps minimise these effects. Patients should be advised to eat smaller, more frequent meals and avoid high-fat foods, which may exacerbate nausea.
More serious but less common side effects include:
-
Pancreatitis: Patients should seek immediate medical attention if they experience severe, persistent abdominal pain that may radiate to the back
-
Gallbladder disease: Rapid weight loss can increase the risk of gallstones; symptoms include right upper abdominal pain, particularly after meals
-
Hypoglycaemia: Particularly when used in combination with insulin or sulfonylureas in patients with type 2 diabetes; dose adjustments of these medications may be needed
-
Diabetic retinopathy complications: Patients with pre-existing diabetic eye disease should have regular ophthalmology follow-up
-
Acute kidney injury: Usually in the context of severe dehydration from gastrointestinal side effects
Mounjaro is not recommended in patients with severe gastrointestinal disease, such as severe gastroparesis. It should not be used during pregnancy or breastfeeding. Women of childbearing potential should use effective contraception, and the medication should be discontinued at least one month before a planned pregnancy. Due to delayed gastric emptying, women using oral contraceptives should use an additional barrier method for 4 weeks after starting tirzepatide and for 4 weeks after each dose increase.
Patients should contact their GP or prescribing clinician if they experience persistent vomiting or diarrhoea leading to dehydration, signs of pancreatitis, visual changes, or any other concerning symptoms. Regular monitoring of weight, glycaemic control (in diabetic patients), and renal function is recommended throughout treatment. For patients with sleep apnoea, it is equally important to maintain regular follow-up with sleep services to assess changes in OSA severity and adjust therapy accordingly.
If you experience any side effects, talk to your healthcare professional. This includes any possible side effects not listed in the package leaflet. You can also report side effects directly via the Yellow Card Scheme at yellowcard.mhra.gov.uk.
Scientific References
- Mounjaro KwikPen 10mg solution for injection in pre-filled pen - Summary of Product Characteristics (SmPC).
- Tirzepatide for managing overweight and obesity. Technology appraisal guidance [TA1026].
- Obstructive sleep apnoea/hypopnoea syndrome and obesity hypoventilation syndrome in over 16s: diagnosis and management. NICE guideline [NG202].
- Tirzepatide for the Treatment of Obstructive Sleep Apnea and Obesity.
- Mounjaro - European Public Assessment Report (EPAR).
Frequently Asked Questions
Can Mounjaro be prescribed specifically for sleep apnoea in the UK?
No, Mounjaro is not licensed for sleep apnoea treatment in the UK. It is prescribed for type 2 diabetes or weight management, and any benefits for obstructive sleep apnoea are secondary to weight reduction achieved through its licensed indications.
Should I stop using my CPAP machine if I start taking Mounjaro?
No, patients should not discontinue CPAP therapy without explicit guidance from their sleep specialist or respiratory physician. CPAP provides immediate therapeutic benefit, whilst weight loss with Mounjaro occurs gradually over months, requiring ongoing monitoring and potential therapy adjustments.
What weight loss results can I expect with Mounjaro for obesity-related sleep apnoea?
Clinical trials show participants achieved significant weight reduction over 52 weeks, with corresponding improvements in apnoea-hypopnoea index. Individual results vary, and treatment should be combined with lifestyle modifications and appropriate OSA-specific therapies under medical supervision.
The health-related content published on this site is based on credible scientific sources and is periodically reviewed to ensure accuracy and relevance. Although we aim to reflect the most current medical knowledge, the material is meant for general education and awareness only.
The information on this site is not a substitute for professional medical advice. For any health concerns, please speak with a qualified medical professional. By using this information, you acknowledge responsibility for any decisions made and understand we are not liable for any consequences that may result.
Heading 1
Heading 2
Heading 3
Heading 4
Heading 5
Heading 6
Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut aliquip ex ea commodo consequat. Duis aute irure dolor in reprehenderit in voluptate velit esse cillum dolore eu fugiat nulla pariatur.
Block quote
Ordered list
- Item 1
- Item 2
- Item 3
Unordered list
- Item A
- Item B
- Item C
Bold text
Emphasis
Superscript
Subscript










