Mounjaro (tirzepatide) is a prescription medicine licensed in the UK for treating type 2 diabetes and chronic weight management in adults with obesity or overweight with weight-related comorbidities. As a dual GIP and GLP-1 receptor agonist, it works by mimicking natural gut hormones that regulate blood glucose and appetite. A common question amongst patients and healthcare professionals is whether Mounjaro functions as an immunosuppressant. Understanding Mounjaro's mechanism of action and its effects—or lack thereof—on the immune system is essential for safe prescribing and informed patient decision-making.
Summary: No, Mounjaro (tirzepatide) is not an immunosuppressant and does not suppress immune function.
- Mounjaro is a dual GIP and GLP-1 receptor agonist licensed for type 2 diabetes and chronic weight management in adults.
- Its mechanism targets metabolic pathways regulating glucose and appetite, not the immune system.
- The MHRA and EMA do not classify tirzepatide as an immunosuppressant in regulatory documentation.
- Patients taking Mounjaro do not typically experience increased susceptibility to infections or impaired wound healing.
- Individuals with autoimmune conditions or taking immunosuppressive medications can generally use Mounjaro safely, though coordinated specialist care is advised.
- Common side effects include gastrointestinal symptoms; serious risks include pancreatitis and gallbladder disease requiring prompt medical attention.
Table of Contents
What Is Mounjaro and How Does It Work?
Mounjaro (tirzepatide) is a prescription medicine licensed in the UK for the treatment of type 2 diabetes mellitus and, more recently, for chronic weight management in adults with obesity (BMI ≥30 kg/m²) or overweight (BMI ≥27 kg/m²) with at least one weight-related comorbidity. It is administered as a once-weekly subcutaneous injection and belongs to a novel class of medications known as dual glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptor agonists.
The mechanism of action of Mounjaro involves mimicking two naturally occurring incretin hormones: GIP and GLP-1. These hormones are released by the gut in response to food intake and play crucial roles in regulating blood glucose levels. GLP-1 receptor activation stimulates insulin secretion from pancreatic beta cells when blood glucose is elevated, suppresses glucagon release (which reduces glucose production by the liver), slows gastric emptying, and promotes satiety. GIP receptor activation also enhances insulin secretion and may have additional effects on fat metabolism and energy balance.
By activating both receptor pathways simultaneously, tirzepatide produces effects that lead to improved glycaemic control, significant weight reduction, and improvements in metabolic risk factors such as blood pressure and lipid profiles. Clinical trials (SURPASS and SURMOUNT programmes) have demonstrated that Mounjaro can reduce HbA1c levels substantially and produce weight loss that varies by dose, typically ranging from 5-20% of body weight depending on the individual, dose and duration of treatment.
It is important to note that Mounjaro is not an immunosuppressant. Its pharmacological actions are targeted specifically at metabolic pathways involving glucose and appetite regulation, not the immune system. Mounjaro is not indicated for the treatment of type 1 diabetes or diabetic ketoacidosis (DKA). Understanding these distinctions is essential for patients and healthcare professionals when considering its use and potential interactions with other conditions or medications.
Mounjaro's Effects on the Immune System
Mounjaro does not function as an immunosuppressant, and there is no official link between tirzepatide and direct suppression of immune function. The Medicines and Healthcare products Regulatory Agency (MHRA) and European Medicines Agency (EMA) do not classify tirzepatide as an immunosuppressant in their regulatory documentation. Immunosuppressants are medications specifically designed to reduce or inhibit the activity of the immune system, commonly used in conditions such as autoimmune diseases, organ transplantation, or inflammatory disorders. Examples include corticosteroids, methotrexate, azathioprine, and biological agents like anti-TNF therapies. Mounjaro's mechanism of action does not involve modulation of immune cell activity, cytokine production, or lymphocyte function.
However, some preclinical and laboratory research into incretin-based therapies has identified potential indirect immunomodulatory effects that may be of academic interest. GLP-1 receptors have been identified not only in pancreatic cells and the gastrointestinal tract but also on various immune cells, including macrophages, lymphocytes, and dendritic cells. Some preclinical studies suggest that GLP-1 receptor agonists may have anti-inflammatory properties, potentially associated with reductions in markers of systemic inflammation. These effects, if clinically relevant, are thought to result primarily from improved metabolic health, weight loss, and reduced adipose tissue inflammation rather than direct immune suppression.
In clinical practice, patients taking Mounjaro do not typically experience increased susceptibility to infections or impaired wound healing—hallmarks of true immunosuppression. While minor infections may be reported among adverse events in clinical trials, these do not occur at rates suggesting immunosuppression. The MHRA and EMA safety monitoring has not identified immunosuppression as a recognised adverse effect of tirzepatide. Nonetheless, as with any medication, individual responses can vary, and ongoing pharmacovigilance continues to monitor the safety profile of Mounjaro in diverse patient populations.
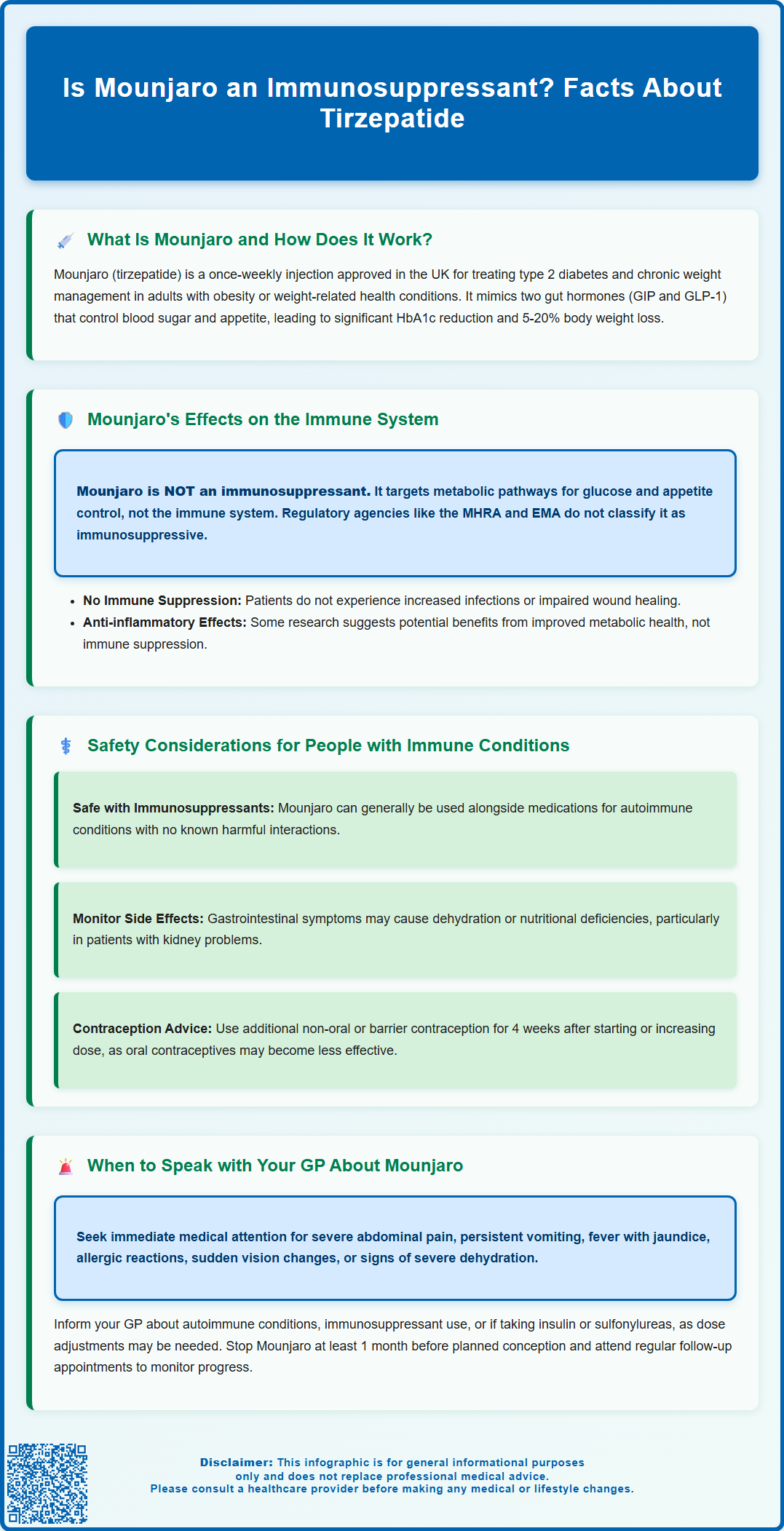
Safety Considerations for People with Immune Conditions
For individuals with pre-existing immune conditions—whether autoimmune diseases (such as rheumatoid arthritis, lupus, or inflammatory bowel disease) or immunodeficiency states—the use of Mounjaro requires careful consideration, though not because it acts as an immunosuppressant. The primary concerns relate to the overall management of complex medical conditions and potential interactions with other treatments.
Patients with autoimmune conditions who are already taking immunosuppressive medications (e.g., corticosteroids, disease-modifying antirheumatic drugs, or biologics) can generally use Mounjaro safely, as there is no known pharmacological interaction that would compromise immune function further. However, these patients often have multiple comorbidities, and the addition of any new medication should be discussed with a specialist to ensure coordinated care.
Individuals with a history of recurrent infections or immunodeficiency should inform their GP or prescribing clinician before starting Mounjaro. While tirzepatide itself does not suppress immunity, gastrointestinal side effects—such as nausea, vomiting, and diarrhoea—are common, particularly during dose escalation. These symptoms could potentially lead to dehydration or nutritional deficiencies if not managed appropriately, which might indirectly affect overall health and resilience. Patients with kidney problems should be monitored, as dehydration can increase the risk of acute kidney injury.
Important safety considerations include:
-
Risk of pancreatitis: Seek urgent medical attention for severe, persistent abdominal pain (which may radiate to the back), with or without vomiting
-
Gallbladder disease: Be aware of symptoms such as right upper quadrant pain, fever, or jaundice
-
Diabetic retinopathy: Patients with pre-existing retinopathy should have appropriate monitoring
-
Hypoglycaemia risk: When used with insulin or sulfonylureas, dose adjustments of these medications may be needed
-
Oral contraceptives: The effectiveness of oral contraceptives may be reduced; consider using a non-oral or barrier method for 4 weeks after starting Mounjaro and after each dose increase
According to NICE guidance, prescribers should conduct a thorough medical history and medication review before initiating Mounjaro. Patients should be counselled on recognising adverse effects and the importance of maintaining adequate hydration and nutrition. There is no evidence to suggest that Mounjaro increases the risk of opportunistic infections or reactivation of latent infections, unlike true immunosuppressants. Nonetheless, individualised risk assessment remains essential, particularly in patients with complex medical backgrounds.
When to Speak with Your GP About Mounjaro
If you are considering Mounjaro for type 2 diabetes or weight management, or if you have already been prescribed this medication, it is important to maintain open communication with your GP or specialist diabetes team. You should arrange a consultation if you have any of the following concerns or circumstances:
-
Pre-existing immune conditions or immunosuppressive therapy: Discuss your full medical history, including autoimmune diseases, recurrent infections, or current use of immunosuppressant medications. Your GP can assess whether Mounjaro is appropriate and coordinate care with relevant specialists.
-
Urgent symptoms requiring immediate medical attention: Severe, persistent abdominal pain (especially if radiating to the back), persistent vomiting, fever with jaundice or right upper abdominal pain, signs of severe allergic reaction (swelling, rash, difficulty breathing), sudden vision changes, or severe dehydration (dizziness, very dark urine, little or no urination).
-
Persistent or severe gastrointestinal symptoms: While nausea, vomiting, and diarrhoea are common side effects, especially when starting or increasing the dose, severe or prolonged symptoms warrant medical review. Dehydration and electrolyte imbalances can occur and may require dose adjustment or additional supportive measures.
-
If you take insulin or sulfonylureas: Discuss potential dose adjustments to reduce the risk of hypoglycaemia (low blood sugar). Know how to recognise and treat hypoglycaemia symptoms.
-
Pregnancy or planning pregnancy: Mounjaro is not recommended during pregnancy or breastfeeding. If you are planning to conceive, Mounjaro should be discontinued at least 1 month before planned conception. If you suspect you may be pregnant, contact your GP immediately to discuss alternative management strategies.
-
If you use oral contraceptives: The effectiveness of oral contraceptives may be reduced when starting Mounjaro or increasing the dose. Consider using a non-oral or barrier method of contraception for 4 weeks after starting treatment and after each dose increase.
-
Signs of infection or unexplained illness: Any new or worsening symptoms should be evaluated promptly. This is standard advice for anyone on long-term medication.
-
Concerns about drug interactions: If you are prescribed new medications or over-the-counter supplements, check with your GP or pharmacist to ensure there are no interactions. Mounjaro can affect gastric emptying, which may alter the absorption of oral medications.
Your GP can provide personalised advice, monitor your response to treatment, and ensure that Mounjaro is used safely and effectively as part of your overall care plan. Regular follow-up appointments are essential to assess glycaemic control, weight loss progress, and any emerging side effects, in line with NICE recommendations for diabetes and obesity management.
If you experience any suspected side effects from Mounjaro, you can report them via the MHRA Yellow Card Scheme (website or app), which helps monitor the safety of medicines in the UK.
Frequently Asked Questions
Does Mounjaro weaken the immune system?
No, Mounjaro does not weaken the immune system. It is not classified as an immunosuppressant by the MHRA or EMA, and patients do not typically experience increased susceptibility to infections.
Can I take Mounjaro if I have an autoimmune condition?
Yes, individuals with autoimmune conditions can generally use Mounjaro safely, as there is no known interaction that compromises immune function. However, discuss your full medical history with your GP or specialist to ensure coordinated care.
What are the main safety concerns with Mounjaro?
Key safety concerns include risk of pancreatitis, gallbladder disease, gastrointestinal side effects, hypoglycaemia when used with insulin or sulfonylureas, and potential effects on diabetic retinopathy. Seek urgent medical attention for severe abdominal pain, persistent vomiting, or signs of allergic reaction.
The health-related content published on this site is based on credible scientific sources and is periodically reviewed to ensure accuracy and relevance. Although we aim to reflect the most current medical knowledge, the material is meant for general education and awareness only.
The information on this site is not a substitute for professional medical advice. For any health concerns, please speak with a qualified medical professional. By using this information, you acknowledge responsibility for any decisions made and understand we are not liable for any consequences that may result.
Heading 1
Heading 2
Heading 3
Heading 4
Heading 5
Heading 6
Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut aliquip ex ea commodo consequat. Duis aute irure dolor in reprehenderit in voluptate velit esse cillum dolore eu fugiat nulla pariatur.
Block quote
Ordered list
- Item 1
- Item 2
- Item 3
Unordered list
- Item A
- Item B
- Item C
Bold text
Emphasis
Superscript
Subscript










