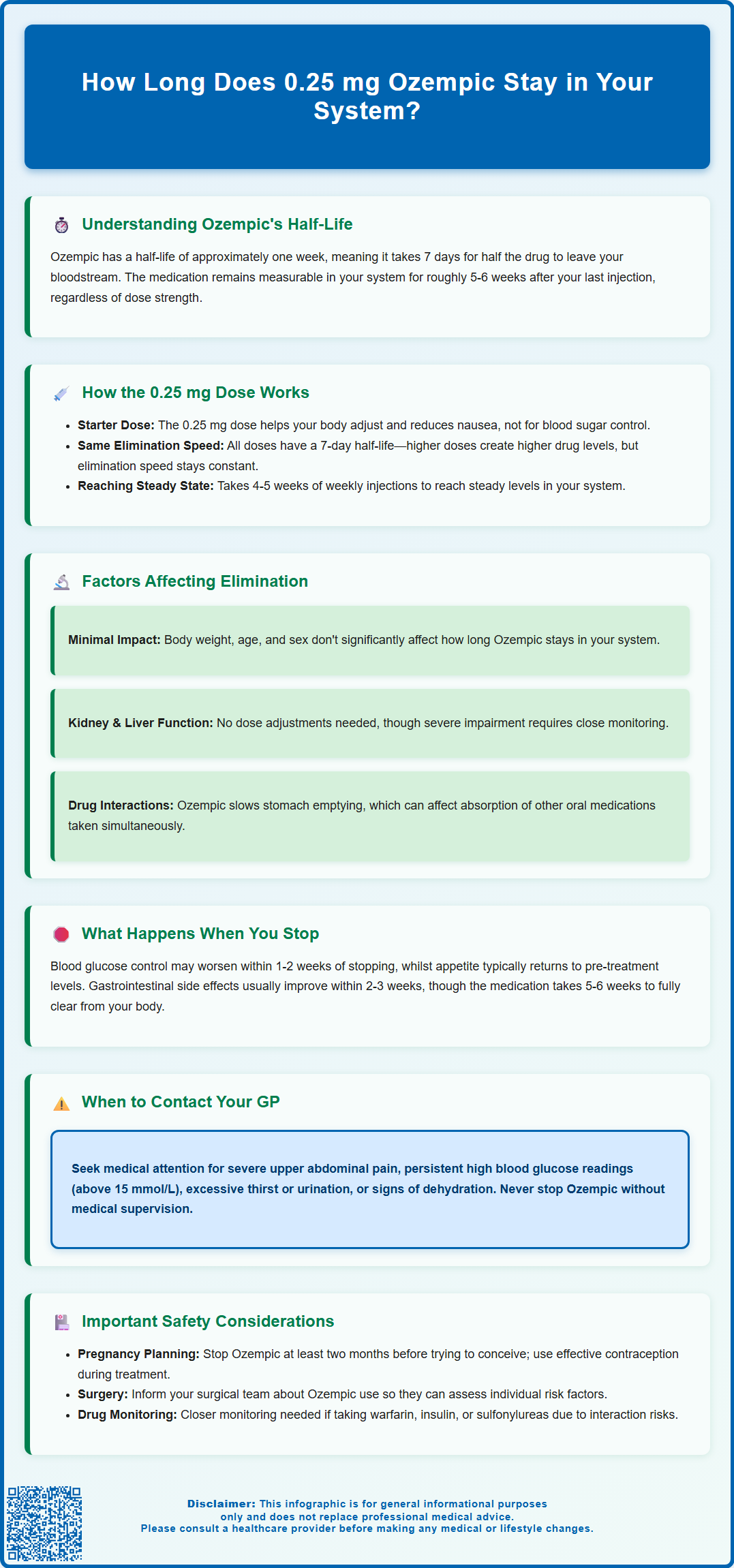
How long does 0.25 mg Ozempic stay in your system? Understanding the duration of semaglutide in your body is essential for managing type 2 diabetes treatment effectively. Ozempic (semaglutide) is a glucagon-like peptide-1 (GLP-1) receptor agonist with an exceptionally long half-life of approximately one week. This means the medication remains detectable in your system for roughly five to six weeks after your final injection, regardless of dose. The 0.25 mg starting dose follows the same elimination pattern as higher maintenance doses. This extended presence allows convenient once-weekly dosing but also means effects—both therapeutic and adverse—persist for several weeks after discontinuation. Understanding this pharmacokinetic profile helps patients and healthcare professionals make informed decisions about treatment changes, side effect management, and planning for surgery or pregnancy.
Summary: Ozempic (semaglutide) 0.25 mg remains detectable in your system for approximately five to six weeks after your final injection, with a half-life of about seven days.
- Semaglutide is a GLP-1 receptor agonist with a half-life of approximately one week, allowing once-weekly dosing for type 2 diabetes management.
- The 0.25 mg dose is an initial titration dose designed to improve tolerability, not for glycaemic control, and elimination time is identical across all doses.
- It takes approximately five half-lives (five to six weeks) for Ozempic to be substantially cleared from the body, regardless of the dose administered.
- Renal or hepatic impairment does not significantly alter elimination times, and no dose adjustments are required based on kidney or liver function.
- Discontinue Ozempic at least two months before planned pregnancy, and inform surgical teams of use to allow appropriate perioperative planning.
- Therapeutic effects including appetite suppression and glucose control gradually diminish over several weeks after stopping, requiring alternative diabetes management strategies.
Table of Contents
Understanding Ozempic's Half-Life and Duration in the Body
Ozempic (semaglutide) is a glucagon-like peptide-1 (GLP-1) receptor agonist licensed in the UK for the treatment of type 2 diabetes mellitus. Understanding how long this medication remains in your system is important for both patients and healthcare professionals, particularly when considering treatment changes or managing potential side effects.
The pharmacokinetic profile of Ozempic is characterised by an exceptionally long half-life of approximately one week (7 days), according to the UK Summary of Product Characteristics (SmPC). The half-life refers to the time it takes for half of the drug concentration in your bloodstream to be eliminated. This extended duration is achieved through specific molecular modifications that slow the medication's breakdown and removal from the body. Semaglutide binds to albumin (a protein in the blood), which protects it from rapid degradation and extends its presence in the circulation.
Based on pharmacological principles, it typically takes five half-lives for a medication to be considered essentially eliminated from the body (approximately 97% cleared). For Ozempic, this means the drug can be measurable in your system for roughly five to six weeks after your final injection, regardless of the dose administered. However, the clinical effects gradually diminish during this period and are generally minimal by the end of this timeframe. This prolonged presence is actually beneficial for treatment adherence, as it allows for once-weekly dosing rather than daily injections. However, it also means that if you experience side effects or need to discontinue treatment, the medication's effects will persist for several weeks after stopping.
How the 0.25 mg Dose Affects Elimination Time
The 0.25 mg dose of Ozempic is specifically designed as an initial titration dose to improve tolerability and is not intended for glycaemic control. According to MHRA-approved prescribing information, patients typically begin with 0.25 mg once weekly for four weeks to allow the body to adjust to the medication and minimise gastrointestinal side effects such as nausea and vomiting.
An important principle to understand is that the elimination half-life remains constant regardless of dose. Whether you receive 0.25 mg, 0.5 mg, 1 mg, or 2 mg, Ozempic will still have a half-life of approximately seven days. What differs between doses is the peak concentration achieved in your bloodstream and the overall drug exposure, not the rate at which your body eliminates the medication. The 0.25 mg dose will reach lower peak concentrations compared to higher maintenance doses, but the time course for elimination follows the same pattern.
During the initial four-week period on 0.25 mg, the medication gradually accumulates in your system until it reaches what is termed 'steady state' – typically achieved after four to five weeks of consistent weekly dosing, as stated in the UK SmPC. This accumulation occurs regardless of dose due to the long half-life of semaglutide. At steady state, the amount of drug being administered each week equals the amount being eliminated, creating a stable concentration. Even at this lower starting dose, if treatment were discontinued, it would still take approximately five to six weeks for the semaglutide to be substantially cleared from your body. This is why healthcare professionals carefully monitor patients during dose escalation and why any side effects experienced may persist even after stopping the initial dose.
Factors That Influence How Long Ozempic Stays in Your System
While the half-life of Ozempic is relatively consistent across most patients, several physiological and clinical factors can influence drug metabolism and elimination to varying degrees.
Renal (kidney) function: According to the UK SmPC, no dose adjustment is required for patients with renal impairment, including those with severe kidney disease. There is limited experience in patients with end-stage renal disease (ESRD) requiring dialysis, so caution is advised when initiating or escalating doses in this population.
Hepatic (liver) function: Similarly, no dose adjustment is required for patients with hepatic impairment, including those with severe liver disease. The liver is involved in the metabolism of the peptide structure, but significant hepatic impairment does not dramatically alter elimination times. Nonetheless, patients with severe liver disease should be monitored more closely when initiating or discontinuing treatment.
Body weight and composition may theoretically influence drug distribution, as semaglutide binds extensively to albumin and distributes into tissues. However, clinical studies have not demonstrated clinically significant differences in half-life based on body mass index (BMI) alone. Age appears to have minimal impact on elimination, with elderly patients showing similar pharmacokinetic profiles to younger adults. Sex does not significantly affect how long Ozempic remains in the system, and no dose adjustments are recommended based on gender.
It is worth noting that drug interactions affecting Ozempic's elimination are uncommon, as it is not metabolised through the cytochrome P450 enzyme system that many oral medications utilise. However, Ozempic does slow gastric emptying, which can affect the absorption of other oral medications taken concurrently. The UK SmPC specifically recommends monitoring INR when initiating semaglutide in patients taking warfarin or other coumarin derivatives. If you take medications with a narrow therapeutic window, such as thyroid medications, discuss potential timing adjustments with your healthcare provider.
What to Expect When Stopping Ozempic Treatment
When discontinuing Ozempic, whether after the initial 0.25 mg dose or a higher maintenance dose, patients should be aware that the medication's effects will not cease immediately. The therapeutic benefits – including appetite suppression, improved glycaemic control, and any weight loss effects – will gradually diminish over the subsequent weeks as drug concentrations decline.
Blood glucose control may begin to deteriorate within one to two weeks after the final injection, though some glucose-lowering effect may persist for up to four weeks due to residual drug levels. Patients with type 2 diabetes should work closely with their GP or diabetes specialist nurse to implement alternative glucose management strategies before stopping treatment. NICE guidelines (NG28) recommend regular blood glucose monitoring during treatment transitions to prevent hyperglycaemia and ensure metabolic stability. Do not stop Ozempic without medical guidance and having an alternative plan for managing your diabetes.
Appetite and weight changes are commonly reported after stopping Ozempic. As the medication's appetite-suppressing effects wane, many patients experience a return of previous hunger patterns, which may lead to weight regain if dietary habits are not maintained. This is not a 'rebound effect' but rather a return to baseline physiology once the drug's influence on GLP-1 receptors diminishes.
Gastrointestinal symptoms that some patients experience during treatment (such as nausea, vomiting, or altered bowel habits) typically improve within two to three weeks of discontinuation, though the extended half-life means these effects may persist longer than with shorter-acting medications. Conversely, some patients report temporary digestive changes as their system readjusts to the absence of the medication.
Patients should contact their GP if they experience significant deterioration in blood glucose control (persistent readings above 15 mmol/L, symptoms of hyperglycaemia such as excessive thirst or urination), unexplained weight changes, or any concerning symptoms after stopping treatment.
Clinical Implications and Safety Considerations
The extended duration of Ozempic in the body carries several important clinical implications that both patients and healthcare professionals should consider when initiating, maintaining, or discontinuing treatment.
Pre-surgical planning: Current UK guidance from the Centre for Perioperative Care (CPOC) and the Association of Anaesthetists indicates that most patients can continue GLP-1 receptor agonists like Ozempic before elective surgery. Standard precautions to manage aspiration risk are typically sufficient. Withholding the medication is generally only necessary in selected high-risk cases or for patients experiencing significant gastrointestinal symptoms. Always inform your surgical team about your Ozempic use so they can make individualised decisions about your care.
Pregnancy planning is another critical consideration. Semaglutide is not recommended during pregnancy, and due to its long washout period, the MHRA advises discontinuing Ozempic at least two months before a planned pregnancy to ensure the medication is cleared from the system. Women of childbearing potential should use effective contraception during treatment and discuss family planning with their healthcare provider well in advance.
Safety monitoring should account for several important risks:
-
Diabetic retinopathy: Patients with existing retinopathy should be monitored closely, particularly if experiencing rapid improvements in blood glucose levels, as this can temporarily worsen eye complications.
-
Gallbladder disease: Semaglutide has been associated with gallstones and related complications. Seek medical advice if you experience upper abdominal pain, particularly after meals.
-
Dehydration and kidney problems: Severe gastrointestinal side effects can lead to dehydration and potential kidney injury. Maintain adequate fluid intake, especially if experiencing vomiting or diarrhoea.
-
Hypoglycaemia: The risk increases when Ozempic is used with insulin or sulfonylureas. Dose adjustments of these medications may be needed.
-
Pancreatitis: Seek urgent medical attention for severe abdominal pain radiating to the back, particularly if accompanied by vomiting.
Drug monitoring and interactions: When initiating Ozempic, patients taking warfarin or other coumarin anticoagulants should have their INR monitored more frequently. The effect on gastric emptying can alter the absorption of other oral medications, so discuss all your medications with your healthcare provider.
If you experience any suspected side effects from Ozempic, report them through the MHRA Yellow Card scheme, which helps monitor medication safety.
Understanding the extended pharmacokinetic profile of Ozempic empowers patients to make informed decisions about their treatment and helps healthcare professionals provide appropriate guidance during all phases of therapy.
Frequently Asked Questions
Does the 0.25 mg dose of Ozempic leave your system faster than higher doses?
No, the elimination half-life remains constant at approximately seven days regardless of dose. The 0.25 mg dose reaches lower peak concentrations but follows the same five to six week elimination timeline as higher maintenance doses.
How long after stopping Ozempic will my blood sugar control be affected?
Blood glucose control may begin to deteriorate within one to two weeks after your final injection, though some glucose-lowering effect may persist for up to four weeks. Work with your GP or diabetes specialist to implement alternative management strategies before discontinuing treatment.
Do I need to stop Ozempic before surgery?
Current UK guidance indicates most patients can continue Ozempic before elective surgery with standard precautions. Withholding is generally only necessary in selected high-risk cases or for patients with significant gastrointestinal symptoms. Always inform your surgical team about your Ozempic use for individualised assessment.
The health-related content published on this site is based on credible scientific sources and is periodically reviewed to ensure accuracy and relevance. Although we aim to reflect the most current medical knowledge, the material is meant for general education and awareness only.
The information on this site is not a substitute for professional medical advice. For any health concerns, please speak with a qualified medical professional. By using this information, you acknowledge responsibility for any decisions made and understand we are not liable for any consequences that may result.
Heading 1
Heading 2
Heading 3
Heading 4
Heading 5
Heading 6
Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut aliquip ex ea commodo consequat. Duis aute irure dolor in reprehenderit in voluptate velit esse cillum dolore eu fugiat nulla pariatur.
Block quote
Ordered list
- Item 1
- Item 2
- Item 3
Unordered list
- Item A
- Item B
- Item C
Bold text
Emphasis
Superscript
Subscript










