Ozempic (semaglutide) is a GLP-1 receptor agonist licensed in the UK for treating type 2 diabetes. As with any medication, patients may wonder about potential side effects, including whether Ozempic causes bladder infections. Understanding the relationship between diabetes medications and urinary tract infections is important for safe and effective treatment. This article examines the evidence linking Ozempic to bladder infections, explores common side effects, and provides guidance on when to seek medical advice whilst taking this medication.
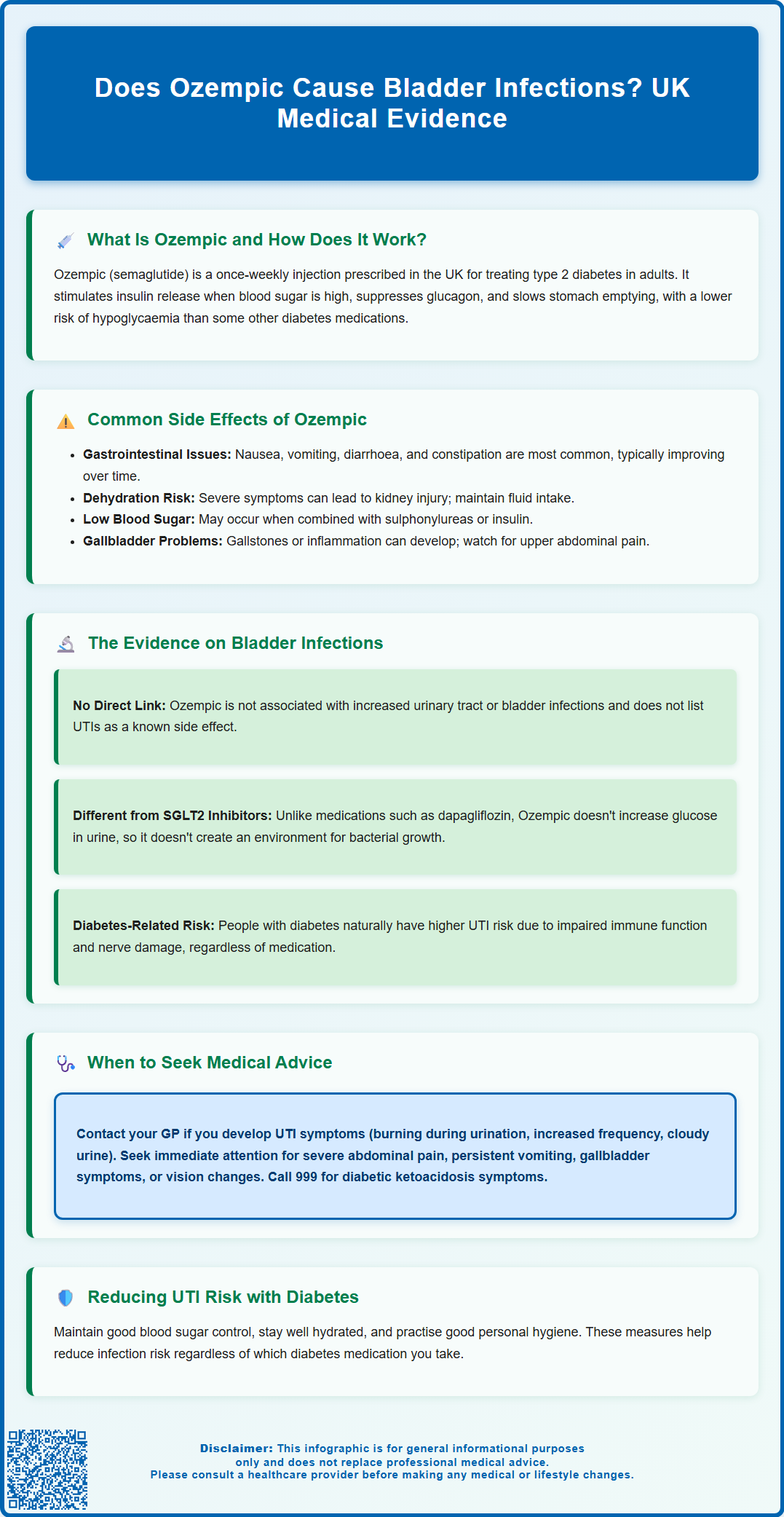
Summary: Ozempic (semaglutide) is not associated with an increased risk of bladder infections according to UK regulatory data and clinical evidence.
- Ozempic is a GLP-1 receptor agonist that works by stimulating insulin secretion, suppressing glucagon, and slowing gastric emptying without altering urinary glucose levels.
- Urinary tract infections are not listed as a known side effect in the MHRA-approved Summary of Product Characteristics for semaglutide.
- SGLT2 inhibitors, not GLP-1 receptor agonists like Ozempic, are the diabetes medication class associated with modestly increased urinary tract infection risk due to increased glucose in urine.
- People with diabetes generally have higher baseline risk of urinary tract infections due to impaired immune function and elevated blood glucose, regardless of medication type.
- Patients should seek medical advice if they develop urinary symptoms such as burning, frequency, urgency, or cloudy urine, which require appropriate investigation and treatment.
- Regular monitoring by diabetes care teams is essential for managing Ozempic treatment and addressing any concerns about side effects or new symptoms.
Table of Contents
What Is Ozempic and How Does It Work?
Ozempic is the brand name for semaglutide, a prescription medication licensed in the United Kingdom for the treatment of type 2 diabetes mellitus in adults. It belongs to a class of medicines called glucagon-like peptide-1 (GLP-1) receptor agonists, which work by mimicking the action of a naturally occurring hormone in the body that helps regulate blood glucose levels. It's important to note that while Ozempic is licensed specifically for type 2 diabetes, Wegovy is the brand name for semaglutide that is licensed for weight management in the UK.
The medication is administered as a once-weekly subcutaneous injection, typically into the abdomen, thigh, or upper arm. Semaglutide works through several complementary mechanisms to improve glycaemic control. It stimulates insulin secretion from the pancreatic beta cells in a glucose-dependent manner, meaning it only triggers insulin release when blood glucose levels are elevated. This reduces the risk of hypoglycaemia compared to some other diabetes medications. Additionally, Ozempic suppresses the release of glucagon, a hormone that raises blood glucose levels, and slows gastric emptying, which helps to moderate the rise in blood glucose after meals.
Beyond its glucose-lowering effects, Ozempic has been shown to promote weight loss in many patients, which can be particularly beneficial for individuals with type 2 diabetes who are overweight or obese. Ozempic is authorised for use alongside diet and exercise to improve blood sugar control. According to NICE guideline NG28 (Type 2 diabetes in adults: management), GLP-1 receptor agonists may be considered when metformin is contraindicated or not tolerated, or when other treatments have not achieved adequate glycaemic control, particularly in patients with a BMI ≥35 kg/m² (or lower in certain ethnic groups) or when weight loss would provide additional clinical benefit.
Common Side Effects of Ozempic
Like all medications, Ozempic can cause side effects, although not everyone experiences them. The most frequently reported adverse effects are gastrointestinal in nature, reflecting the medication's action on the digestive system. These include nausea, vomiting, diarrhoea, abdominal pain, and constipation. These symptoms are typically most pronounced when starting treatment or increasing the dose, and often diminish over time as the body adjusts to the medication. To minimise gastrointestinal side effects, healthcare professionals usually initiate treatment at a low dose and gradually increase it over several weeks.
Severe or persistent gastrointestinal symptoms can lead to dehydration, which may increase the risk of acute kidney injury. Patients should maintain adequate fluid intake and seek prompt medical advice if unable to keep fluids down or experiencing persistent vomiting or diarrhoea.
Other common side effects include injection site reactions such as redness, itching, or swelling at the site where the medication is administered. Some patients may experience fatigue, dizziness, or headaches, particularly during the initial weeks of treatment. Hypoglycaemia, whilst less common with GLP-1 receptor agonists used alone, can occur when Ozempic is combined with other glucose-lowering medications such as sulphonylureas or insulin. Patients taking these combinations should be aware of the signs of low blood sugar, including trembling, sweating, confusion, and palpitations.
Gallbladder problems, including gallstones (cholelithiasis) and inflammation of the gallbladder (cholecystitis), have been reported with semaglutide use. Symptoms may include right upper abdominal pain, fever, nausea, vomiting, or jaundice, and require prompt medical assessment.
More serious but rare adverse effects include pancreatitis, which presents with severe abdominal pain that may radiate to the back. Changes in vision related to diabetic retinopathy may occur, particularly in patients with pre-existing retinopathy and those experiencing rapid improvements in blood glucose control. The MHRA advises appropriate monitoring, especially for patients with a history of diabetic retinopathy, and any concerning symptoms should be reported promptly to a healthcare professional. The benefits of improved glycaemic control typically outweigh the risks for most patients when the medication is used appropriately under medical supervision.
Urinary Tract Infections and Diabetes Medications
Concerns about urinary tract infections, including bladder infections (cystitis), have been raised in relation to certain diabetes medications, particularly sodium-glucose co-transporter-2 (SGLT2) inhibitors such as dapagliflozin and empagliflozin. These medications work by increasing glucose excretion in the urine, which can create an environment more conducive to bacterial growth. While SGLT2 inhibitors are associated with a modestly increased risk of urinary tract infections in some patients, genital mycotic infections (thrush) are actually the more characteristic and common infection risk with this class of medications.
In contrast, there is no established clinical link between GLP-1 receptor agonists like Ozempic and an increased risk of urinary tract or bladder infections. According to the Summary of Product Characteristics for Ozempic approved by the MHRA and the European Medicines Agency's European Public Assessment Report (EPAR), urinary tract infections are not listed as a known side effect of semaglutide. The mechanism of action of semaglutide does not involve altering glucose levels in the urine, which is the primary reason SGLT2 inhibitors may increase infection risk.
That said, people with diabetes in general have a higher baseline risk of developing urinary tract infections compared to the non-diabetic population. This increased susceptibility is related to several factors, including impaired immune function associated with poorly controlled blood glucose, incomplete bladder emptying due to diabetic autonomic neuropathy, and the presence of glucose in the urine when blood sugar levels are elevated. Therefore, whilst Ozempic itself does not appear to cause bladder infections, patients with diabetes should remain vigilant about urinary symptoms regardless of which medications they are taking. Maintaining good glycaemic control, staying well hydrated, and practising good personal hygiene can help reduce the risk of urinary tract infections in people with diabetes, as advised by NICE guidance on urinary tract infections (NG109).
When to Seek Medical Advice While Taking Ozempic
Patients taking Ozempic should be aware of situations that warrant prompt medical attention. If you develop symptoms suggestive of a urinary tract infection—such as burning or pain when passing urine, increased urinary frequency, urgency, cloudy or foul-smelling urine, lower abdominal discomfort, or blood in the urine—you should contact your GP. Whilst these symptoms are not directly caused by Ozempic, they require appropriate investigation and treatment, typically with antibiotics if a bacterial infection is confirmed. If you develop symptoms of pyelonephritis (kidney infection) such as fever, back pain, and feeling generally unwell, contact your GP for same-day assessment or call NHS 111 if your GP is unavailable.
Severe or persistent gastrointestinal symptoms whilst taking Ozempic should also prompt medical review. If you experience severe abdominal pain, particularly if it radiates to your back and is accompanied by nausea and vomiting, this could indicate pancreatitis and requires immediate medical attention via your GP, NHS 111, or 999 in severe cases. Persistent vomiting or diarrhoea can lead to dehydration and potential kidney problems, which is particularly concerning for people with diabetes, and may necessitate temporary adjustment of your diabetes medications.
If you develop symptoms suggestive of gallbladder problems—such as severe pain in the upper right abdomen, fever, nausea, vomiting, or yellowing of the skin or eyes (jaundice)—seek urgent medical advice from your GP or NHS 111.
Signs of hypoglycaemia, especially if recurrent or severe, should be discussed with your diabetes care team, as your treatment regimen may need adjustment. Any sudden changes in vision, symptoms of allergic reaction such as rash, swelling of the face or throat, or difficulty breathing require urgent medical assessment.
Diabetic ketoacidosis (DKA)—with symptoms including excessive thirst, frequent urination, nausea, vomiting, abdominal pain, confusion, or fruity-smelling breath—requires emergency medical attention via 999. While DKA is uncommon in type 2 diabetes and not a direct effect of GLP-1 receptor agonists like Ozempic, it can occur in certain situations, particularly if you are also taking SGLT2 inhibitors or insulin. Regular follow-up with your diabetes care team is essential to monitor your response to Ozempic, adjust dosing as needed, and address any concerns about side effects or symptoms that may arise during treatment.
Scientific References
- Ozempic 0.5 mg solution for injection in pre-filled pen - Summary of Product Characteristics (SmPC).
- Ozempic - European Public Assessment Report (EPAR).
- Type 2 diabetes in adults: management. NICE guideline NG28.
- Urinary tract infection (lower): antimicrobial prescribing. NICE guideline NG109.
- Urinary tract infections (UTIs).
- Diagnosis of urinary tract infections: quick reference tools for primary care.
Frequently Asked Questions
Can Ozempic increase my risk of getting a urinary tract infection?
No, Ozempic (semaglutide) is not associated with an increased risk of urinary tract infections according to UK regulatory data. Unlike SGLT2 inhibitors, Ozempic does not increase glucose in the urine, which is the mechanism that can promote bacterial growth.
What are the most common side effects of Ozempic?
The most common side effects of Ozempic are gastrointestinal, including nausea, vomiting, diarrhoea, abdominal pain, and constipation. These symptoms typically occur when starting treatment or increasing the dose and often improve over time as the body adjusts to the medication.
When should I contact my doctor while taking Ozempic?
Contact your GP if you experience severe abdominal pain (which may indicate pancreatitis), persistent vomiting or diarrhoea, symptoms of gallbladder problems, recurrent hypoglycaemia, sudden vision changes, or any signs of urinary tract infection such as burning when urinating or cloudy urine.
The health-related content published on this site is based on credible scientific sources and is periodically reviewed to ensure accuracy and relevance. Although we aim to reflect the most current medical knowledge, the material is meant for general education and awareness only.
The information on this site is not a substitute for professional medical advice. For any health concerns, please speak with a qualified medical professional. By using this information, you acknowledge responsibility for any decisions made and understand we are not liable for any consequences that may result.
Heading 1
Heading 2
Heading 3
Heading 4
Heading 5
Heading 6
Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut aliquip ex ea commodo consequat. Duis aute irure dolor in reprehenderit in voluptate velit esse cillum dolore eu fugiat nulla pariatur.
Block quote
Ordered list
- Item 1
- Item 2
- Item 3
Unordered list
- Item A
- Item B
- Item C
Bold text
Emphasis
Superscript
Subscript












