Can you take Qsymia and Wegovy together for weight loss? This question arises as patients seek more effective weight management solutions. Qsymia (phentermine-topiramate) and Wegovy (semaglutide) work through different mechanisms—central nervous system stimulation versus GLP-1 receptor activation. However, Qsymia is not licensed in the UK, and no clinical evidence supports combining these medications. UK guidance from NICE and the MHRA does not recommend combination weight loss pharmacotherapy. This article examines the safety concerns, regulatory position, and evidence-based alternatives to help you understand appropriate treatment options within the UK healthcare system.
Summary: No, there is no clinical evidence or UK regulatory approval supporting the concurrent use of Qsymia and Wegovy for weight management.
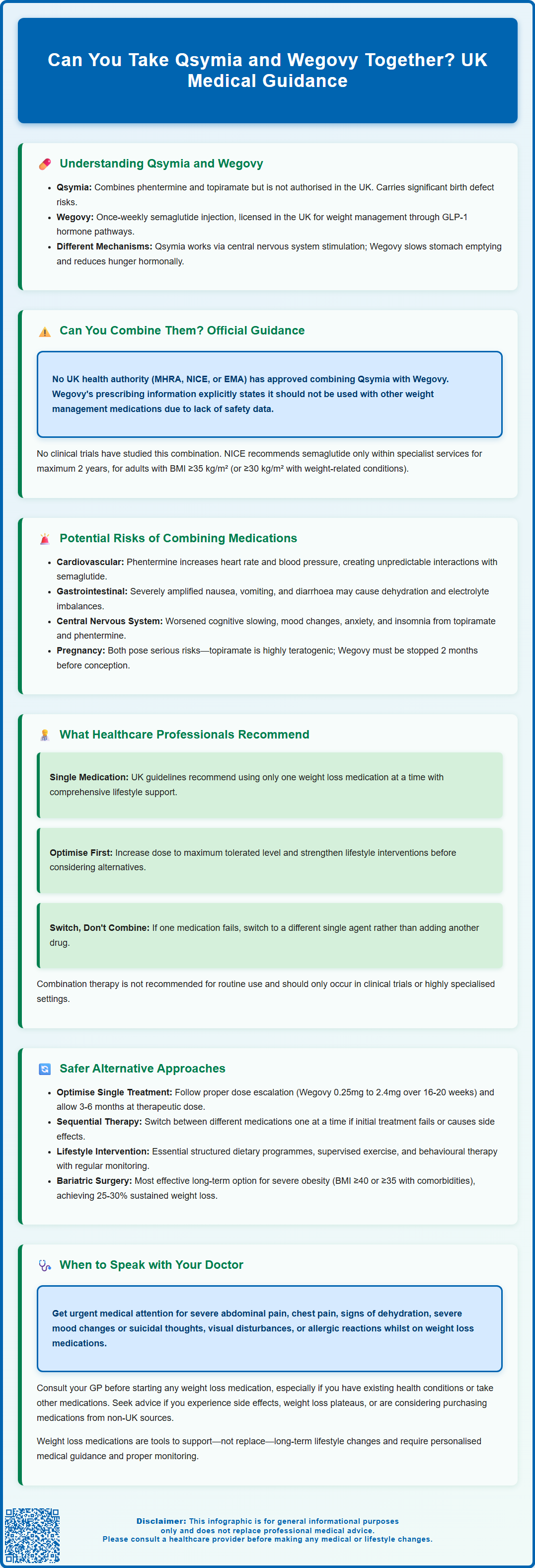
- Qsymia (phentermine-topiramate) is not licensed for use in the UK by the MHRA
- Wegovy (semaglutide) is a GLP-1 receptor agonist licensed in the UK and should not be combined with other weight management products according to its Summary of Product Characteristics
- No clinical trials have investigated the safety or efficacy of combining these two medications
- Potential risks include cardiovascular effects, amplified gastrointestinal side effects, and central nervous system disturbances
- UK guidance from NICE recommends single-agent pharmacotherapy optimised within specialist weight management services
- Sequential therapy or bariatric surgery referral are evidence-based alternatives for patients not responding to single-agent treatment
Table of Contents
- Understanding Qsymia and Wegovy: Different Mechanisms for Weight Loss
- Can You Take Qsymia and Wegovy Together? Current Medical Guidance
- Potential Risks and Drug Interactions When Combining Weight Loss Medications
- What Healthcare Professionals Recommend for Combination Weight Management
- Alternative Approaches: Sequential or Single-Agent Therapy
- When to Speak with Your Doctor About Weight Loss Treatment Options
- Frequently Asked Questions
Understanding Qsymia and Wegovy: Different Mechanisms for Weight Loss
Qsymia and Wegovy represent two distinct pharmacological approaches to weight management, each working through different mechanisms within the body. Understanding these differences is essential when considering their use, either individually or in combination.
Qsymia is a combination medication containing phentermine and topiramate extended-release. Phentermine acts as a sympathomimetic amine, suppressing appetite through effects on the central nervous system by increasing noradrenaline release. Topiramate, originally developed as an anticonvulsant, contributes to weight loss through multiple mechanisms including appetite suppression and increased satiety. It is important to note that Qsymia is not currently authorised for use in the UK by the Medicines and Healthcare products Regulatory Agency (MHRA). Furthermore, phentermine is not licensed or marketed in the UK. Topiramate is licensed in the UK for epilepsy and migraine prophylaxis, and any use for weight management would be off-label in specialist settings only. Topiramate carries significant risks, particularly its teratogenic effects, requiring strict adherence to the MHRA Pregnancy Prevention Programme for women of childbearing potential.
Wegovy (semaglutide 2.4mg) is a glucagon-like peptide-1 (GLP-1) receptor agonist licensed in the UK for weight management. It mimics the action of the naturally occurring hormone GLP-1, which regulates appetite and food intake. Semaglutide works by slowing gastric emptying, increasing feelings of fullness, and acting on appetite centres in the brain to reduce hunger. Wegovy is administered as a once-weekly subcutaneous injection and has demonstrated significant weight loss in clinical trials when combined with lifestyle modifications.
These medications operate through fundamentally different pathways—Qsymia primarily through central nervous system stimulation and metabolic effects, whilst Wegovy works through hormonal appetite regulation. According to the UK Summary of Product Characteristics (SmPC), Wegovy should not be used in combination with other GLP-1 receptor agonists or other weight management products. This distinction becomes particularly relevant when considering whether combining such treatments might offer additional benefits or pose unnecessary risks.
Can You Take Qsymia and Wegovy Together? Current Medical Guidance
The question of combining Qsymia and Wegovy lacks robust clinical evidence, and there is no official guidance supporting their concurrent use. Neither the MHRA, the National Institute for Health and Care Excellence (NICE), nor the European Medicines Agency (EMA) has evaluated or approved this specific combination for weight management.
From a UK perspective, the question is somewhat theoretical given that Qsymia is not licensed for use in this country. NICE guidance for weight management (CG189) recommends pharmacological interventions as part of a multicomponent strategy, but they focus on licensed medications used individually at appropriate doses. For semaglutide 2.4mg specifically, NICE technology appraisal guidance (TA875) recommends its use only within specialist weight management services, for a maximum duration of 2 years, and for adults with a BMI of at least 35 kg/m² (or at least 30 kg/m² with weight-related comorbidities). The guidance does not address combination therapy with multiple weight loss agents.
Clinical trials have not specifically investigated the safety or efficacy of combining these two medications. The absence of such evidence means that healthcare professionals cannot reliably predict how these drugs might interact, whether they would provide additive benefits, or what adverse effects might emerge from their concurrent use. In evidence-based medicine, the lack of clinical trial data represents a significant barrier to recommending combination therapy.
Importantly, the UK SmPC for Wegovy explicitly states that it should not be used with other weight management medicines. This regulatory position reflects the absence of safety and efficacy data for such combinations. Without specific safety data, most clinicians would exercise considerable caution before prescribing such a combination, and many would advise against it entirely in favour of optimising single-agent therapy first.
Potential Risks and Drug Interactions When Combining Weight Loss Medications
Combining weight loss medications carries inherent risks that extend beyond the known side effect profiles of individual drugs. When considering Qsymia and Wegovy together, several potential safety concerns warrant careful consideration.
Cardiovascular effects represent a primary concern. Phentermine, a component of Qsymia, is a sympathomimetic agent that can increase heart rate and blood pressure. Whilst GLP-1 receptor agonists like semaglutide typically have neutral or beneficial cardiovascular effects in clinical trials, the combination with a stimulant medication could theoretically alter this profile. Patients with pre-existing cardiovascular conditions, hypertension, or arrhythmias would face particular risks. The cumulative effect on heart rate and blood pressure has not been studied and could prove unpredictable.
Gastrointestinal side effects could be significantly amplified. Wegovy commonly causes nausea, vomiting, diarrhoea, and constipation, particularly during dose escalation. Topiramate (in Qsymia) can also cause gastrointestinal disturbances. Combining these medications might lead to intolerable digestive symptoms, potentially resulting in dehydration, electrolyte imbalances, or poor nutritional intake—outcomes that would be counterproductive to healthy weight management.
Central nervous system effects require consideration as well. Both medications can affect mood and cognition. Topiramate is associated with cognitive slowing, difficulty concentrating, mood changes, and metabolic acidosis. Phentermine can cause insomnia, anxiety, and restlessness. Whilst semaglutide's CNS effects are generally limited to appetite regulation, the combination could potentially exacerbate psychiatric symptoms or create new ones.
Gallbladder disease is a recognised risk with GLP-1 receptor agonists like Wegovy. Symptoms such as right upper quadrant pain, fever, or jaundice require urgent medical attention. This risk might be compounded by rapid weight loss from combination therapy.
For people with diabetes taking insulin or sulfonylureas, starting a GLP-1 receptor agonist requires careful monitoring and potential dose adjustments of existing medications to prevent hypoglycaemia.
Pregnancy risks are significant. Topiramate is highly teratogenic and subject to a strict MHRA Pregnancy Prevention Programme. Wegovy is not recommended during pregnancy and should be discontinued at least two months before a planned pregnancy.
If you experience any suspected side effects from medication, report them to the MHRA Yellow Card scheme, which helps monitor medicine safety.
What Healthcare Professionals Recommend for Combination Weight Management
Healthcare professionals in the UK follow evidence-based guidelines that prioritise single-agent pharmacotherapy optimised within a comprehensive weight management programme. The approach recommended by NICE and supported by UK clinical practice emphasises a structured, stepwise strategy rather than combining multiple weight loss medications.
The foundation of any weight management plan involves lifestyle modifications, including dietary changes, increased physical activity, and behavioural support. Pharmacological interventions are considered adjuncts to—not replacements for—these fundamental approaches. When medication is appropriate, clinicians typically begin with a single licensed agent, carefully titrating the dose and monitoring response over several months before considering any changes.
For patients who might benefit from pharmacotherapy, GLP-1 receptor agonists like Wegovy are increasingly favoured due to their robust evidence base, including cardiovascular safety data and significant weight loss outcomes in clinical trials. NICE technology appraisal guidance (TA875) recommends semaglutide 2.4mg for adults with a BMI of 35 kg/m² or greater, or 30 kg/m² or greater with at least one weight-related comorbidity, when provided alongside a reduced-calorie diet and increased physical activity. Importantly, this treatment must be provided by specialist weight management services (Tier 3) and is limited to a maximum duration of 2 years.
If initial therapy proves insufficient, healthcare professionals typically reassess the overall treatment plan rather than adding another weight loss medication. This might involve:
-
Optimising the current medication dose to the maximum tolerated level
-
Intensifying lifestyle interventions with specialist dietetic or psychological support
-
Switching to an alternative single agent if the first medication is poorly tolerated or ineffective
-
Considering bariatric surgery referral for patients meeting eligibility criteria who have not achieved adequate weight loss with medical management
The consensus among UK specialists is that combination pharmacotherapy for weight loss should only be considered within clinical trials or highly specialised settings where close monitoring is possible. The potential risks, absence of safety data, and availability of effective single-agent options make combination therapy an inappropriate choice for routine clinical practice.
Alternative Approaches: Sequential or Single-Agent Therapy
Rather than combining weight loss medications, evidence-based practice supports sequential therapy or optimised single-agent treatment as safer and more practical approaches to weight management.
Single-agent optimisation represents the first-line strategy. For patients prescribed Wegovy in the UK, this involves following the recommended dose escalation schedule, starting at 0.25mg weekly and gradually increasing to the maintenance dose of 2.4mg over 16-20 weeks. This gradual titration minimises side effects whilst allowing the body to adapt to the medication. Many patients achieve significant weight loss—typically 10-15% of body weight or more—with semaglutide alone when combined with appropriate lifestyle modifications, as demonstrated in the STEP clinical trial programme. Ensuring adequate time at the therapeutic dose (at least 3-6 months) before deeming treatment unsuccessful is essential, as weight loss continues progressively over time.
Sequential therapy involves trying different medications one at a time rather than simultaneously. If a patient does not respond adequately to one agent or experiences intolerable side effects, switching to an alternative medication with a different mechanism of action may prove beneficial. For example, a patient who cannot tolerate or does not respond to a GLP-1 receptor agonist might be switched to naltrexone-bupropion or orlistat, depending on individual circumstances and contraindications.
Comprehensive lifestyle intervention remains paramount regardless of pharmacological choices. This includes:
-
Structured dietary programmes with regular dietetic input
-
Supervised exercise regimens tailored to individual capabilities
-
Behavioural therapy addressing eating patterns and psychological factors
-
Regular monitoring of weight, metabolic parameters, and medication adherence
For patients with severe obesity (BMI ≥40 kg/m² or ≥35 kg/m² with comorbidities) who have not achieved adequate weight loss with optimal medical management, bariatric surgery offers the most effective long-term solution. Procedures such as gastric bypass or sleeve gastrectomy typically result in sustained weight loss of 25-30% of total body weight and improvement in obesity-related conditions. NICE provides clear criteria for bariatric surgery referral, including lower BMI thresholds for people of Asian family origin and expedited pathways for those with recent-onset type 2 diabetes. Patients require assessment by a Tier 3 multidisciplinary team before referral to Tier 4 surgical services. This option should be discussed with appropriate patients rather than pursuing unproven medication combinations.
When to Speak with Your Doctor About Weight Loss Treatment Options
Knowing when to seek medical advice about weight management is crucial for safe and effective treatment. You should consult your GP or healthcare provider if you are considering any pharmacological intervention for weight loss, particularly if you are already taking medications or have existing health conditions.
Specific situations requiring medical consultation include:
-
Before starting any weight loss medication, including those purchased online or from overseas sources. Your doctor needs to assess your suitability, screen for contraindications, and ensure appropriate monitoring
-
If you are currently taking Wegovy or any weight loss medication and considering adding another agent. Never combine prescription medications without explicit medical guidance
-
When experiencing side effects from weight loss medications, particularly severe nausea, vomiting, abdominal pain, changes in heart rate, mood disturbances, or any concerning symptoms
-
If weight loss has plateaued after several months on medication, as your treatment plan may need adjustment
-
Before purchasing medications from non-UK sources, as these may not meet MHRA safety standards and could be counterfeit or inappropriate
-
If you have diabetes and take insulin or sulfonylureas, as starting a GLP-1 receptor agonist may require adjustment of these medications to prevent hypoglycaemia
-
If you are pregnant, breastfeeding, or planning pregnancy. Wegovy is not recommended during pregnancy and should be discontinued at least 2 months before planned conception. Topiramate is highly teratogenic and requires strict adherence to pregnancy prevention measures
Red flag symptoms requiring urgent medical attention include:
-
Severe, persistent abdominal pain (which could indicate pancreatitis)
-
Right upper quadrant pain, fever, or jaundice (possible gallbladder problems)
-
Signs of dehydration (dizziness, reduced urination, extreme thirst)
-
Chest pain, palpitations, or significant breathlessness
-
Severe mood changes, depression, or suicidal thoughts
-
Visual disturbances or severe headaches
-
Allergic reactions (rash, swelling, difficulty breathing)
Your doctor can provide personalised advice based on your medical history, current medications, weight loss goals, and individual risk factors. They can discuss licensed treatment options available in the UK, refer you to specialist weight management services if appropriate, and ensure any pharmacological intervention is part of a comprehensive, evidence-based plan. Remember that sustainable weight loss typically requires long-term commitment to lifestyle changes, and medications serve as tools to support—not replace—these fundamental modifications.
If you experience any suspected side effects from medication, report them to the MHRA Yellow Card scheme. Open, honest communication with your healthcare team is essential for achieving safe and effective weight management outcomes.
Frequently Asked Questions
Is Qsymia available in the UK?
No, Qsymia is not currently authorised for use in the UK by the MHRA. Phentermine, one of its components, is not licensed or marketed in the UK, and topiramate is only licensed for epilepsy and migraine prophylaxis.
What does NICE recommend for weight loss medication?
NICE recommends single-agent pharmacotherapy as part of a comprehensive weight management programme within specialist services. For semaglutide 2.4mg (Wegovy), NICE guidance TA875 recommends use for adults with BMI ≥35 kg/m² or ≥30 kg/m² with comorbidities, for a maximum of 2 years.
What should I do if weight loss plateaus on Wegovy?
Consult your healthcare provider to reassess your treatment plan. Options include optimising lifestyle interventions, ensuring adequate time at therapeutic dose, switching to an alternative medication, or considering bariatric surgery referral if you meet eligibility criteria.
The health-related content published on this site is based on credible scientific sources and is periodically reviewed to ensure accuracy and relevance. Although we aim to reflect the most current medical knowledge, the material is meant for general education and awareness only.
The information on this site is not a substitute for professional medical advice. For any health concerns, please speak with a qualified medical professional. By using this information, you acknowledge responsibility for any decisions made and understand we are not liable for any consequences that may result.
Heading 1
Heading 2
Heading 3
Heading 4
Heading 5
Heading 6
Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut aliquip ex ea commodo consequat. Duis aute irure dolor in reprehenderit in voluptate velit esse cillum dolore eu fugiat nulla pariatur.
Block quote
Ordered list
- Item 1
- Item 2
- Item 3
Unordered list
- Item A
- Item B
- Item C
Bold text
Emphasis
Superscript
Subscript










