Combining berberine and Saxenda together is a topic of growing interest amongst individuals seeking enhanced weight management support. Saxenda (liraglutide 3mg) is a prescription-only GLP-1 receptor agonist licensed by the MHRA for weight management, whilst berberine is a plant-derived alkaloid sold as a food supplement with potential metabolic effects. Both substances influence glucose regulation and appetite through different mechanisms, but no official UK guidance addresses their concurrent use. This article examines the pharmacological considerations, potential interactions, safety concerns, and clinical evidence surrounding this combination, emphasising the importance of medical supervision before using berberine alongside Saxenda.
Summary: There is no official UK guidance on taking berberine and Saxenda together, and the combination should only be considered under medical supervision due to potential additive effects on glucose metabolism and gastrointestinal tolerability.
- Saxenda (liraglutide 3mg) is a prescription GLP-1 receptor agonist licensed by the MHRA for weight management, whilst berberine is an unlicensed food supplement with potential metabolic effects.
- Both substances influence glucose regulation through different mechanisms, raising theoretical concerns about additive hypoglycaemia risk, particularly in individuals with diabetes.
- Combining berberine and Saxenda may intensify gastrointestinal side effects including nausea, vomiting, and diarrhoea, potentially affecting treatment tolerability.
- No clinical trials have investigated the safety or efficacy of this combination, and berberine supplements lack the quality controls applied to licensed medicines.
- Patients should consult their GP or prescribing clinician before combining these substances and report any suspected adverse effects through the MHRA Yellow Card scheme.
Table of Contents
Understanding Berberine and Saxenda: Mechanisms and Uses
Berberine is a naturally occurring alkaloid compound extracted from various plants, including Berberis species. Traditionally used in Chinese and Ayurvedic medicine, berberine has gained attention in recent years for its potential metabolic effects. Research suggests it may influence glucose metabolism through activation of AMP-activated protein kinase (AMPK), a cellular enzyme that plays a crucial role in energy balance. Some studies indicate berberine may help improve insulin sensitivity and support modest weight management, though it is not licensed as a medicine in the UK and is typically sold as a food supplement.
Saxenda (liraglutide 3mg) is a prescription-only medicine licensed by the MHRA for weight management in adults with a body mass index (BMI) of 30 kg/m² or greater, or 27 kg/m² or greater with weight-related comorbidities such as dysglycaemia (including prediabetes or type 2 diabetes), hypertension, dyslipidaemia, or obstructive sleep apnoea. Saxenda is used as an adjunct to a reduced-calorie diet and increased physical activity. It belongs to a class of medications called glucagon-like peptide-1 (GLP-1) receptor agonists and is administered by subcutaneous injection, with weekly dose titration to reach the maintenance dose of 3mg once daily.
Saxenda works by mimicking the action of the naturally occurring hormone GLP-1, which regulates appetite and food intake. By activating GLP-1 receptors in the brain, Saxenda helps reduce hunger, increase feelings of fullness, and slow gastric emptying, leading to reduced calorie intake and gradual weight loss. Treatment should be discontinued if at least 5% weight loss has not been achieved after 12 weeks at the 3mg daily dose.
Both substances influence metabolic pathways related to glucose regulation and weight management, but through different mechanisms. Saxenda is a rigorously tested pharmaceutical product with established efficacy and safety profiles, whilst berberine remains a dietary supplement with variable quality and limited regulatory oversight in the UK. Understanding these fundamental differences is essential when considering their combined use.
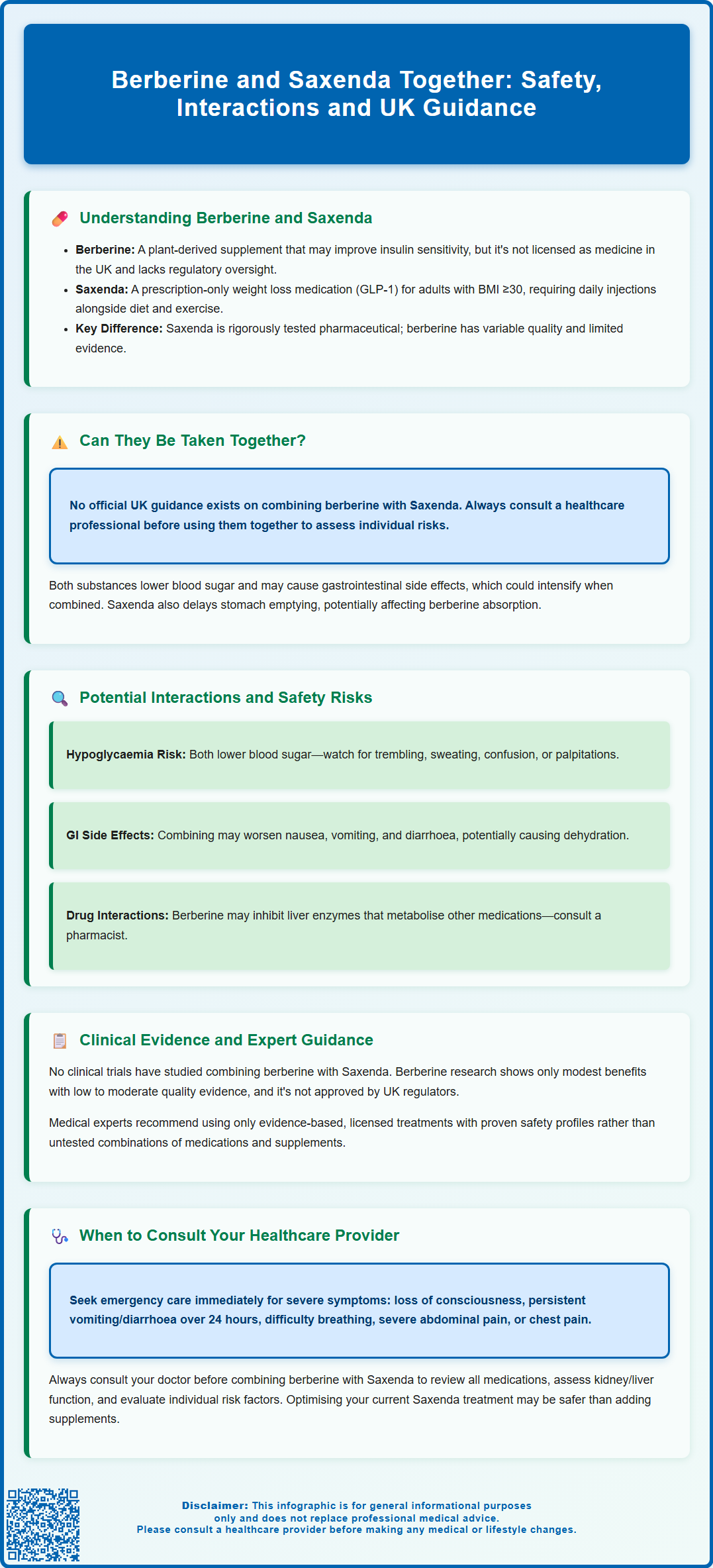
Can Berberine and Saxenda Be Taken Together?
There is currently no official guidance from UK regulatory bodies such as the MHRA, NICE, or the NHS specifically addressing the concurrent use of berberine and Saxenda. The absence of formal contraindications does not automatically mean the combination is safe or advisable. The decision to use these substances together should be made only after thorough discussion with a qualified healthcare professional who can assess individual circumstances, medical history, and potential risks.
From a pharmacological perspective, both berberine and Saxenda influence glucose metabolism and may affect blood sugar levels. Saxenda's prescribing information does not list berberine as a specific interaction, largely because dietary supplements are not routinely included in pharmaceutical interaction databases. However, the theoretical concern centres on the additive effects on glucose regulation. The risk of hypoglycaemia (low blood sugar) with Saxenda alone is relatively low, but increases when used alongside insulin or sulfonylureas. Berberine's glucose-lowering properties could potentially compound this risk in some individuals.
Additionally, both substances may cause gastrointestinal side effects. Saxenda commonly causes nausea, vomiting, diarrhoea, and constipation, especially during the initial titration period. Berberine is also associated with digestive disturbances, including diarrhoea, constipation, and abdominal discomfort. Taking them together could potentially intensify these adverse effects, making treatment less tolerable and affecting adherence.
Saxenda's effect on delaying gastric emptying may also influence the absorption of some oral medicines, which could be relevant when taking berberine or other medications, particularly those with a narrow therapeutic index.
It is crucial to recognise that berberine supplements in the UK are not subject to the same rigorous quality controls as licensed medicines. Variability in berberine content, purity, and the presence of undeclared ingredients may pose additional, unpredictable risks when combined with prescription medications like Saxenda. If you experience any suspected side effects, report them to the MHRA through the Yellow Card scheme.
Potential Interactions and Safety Considerations
When considering the combination of berberine and Saxenda, several safety considerations warrant careful attention. Firstly, berberine has been shown in some laboratory and animal studies to inhibit certain cytochrome P450 enzymes, particularly CYP3A4 and CYP2D6, which are involved in the metabolism of many medications. While clinical data on these interactions in humans is limited, there is potential for berberine to affect drug metabolism. Whilst Saxenda (liraglutide) is primarily metabolised through proteolytic degradation rather than hepatic cytochrome pathways, the broader implications of enzyme inhibition could affect other medications an individual may be taking concurrently. A pharmacist can help check for potential interactions using resources like the BNF.
Hypoglycaemia risk represents a significant concern, particularly for individuals with type 2 diabetes. Saxenda is known to increase the risk of hypoglycaemia when used alongside insulin or insulin secretagogues (such as sulphonylureas). Berberine has demonstrated glucose-lowering properties in clinical studies, which could theoretically compound this risk. Symptoms of hypoglycaemia include trembling, sweating, confusion, palpitations, and in severe cases, loss of consciousness. Patients must be educated to recognise these symptoms and take appropriate action.
The gastrointestinal tolerability of the combination also requires consideration. Both substances commonly cause nausea and altered bowel habits. Severe or persistent gastrointestinal symptoms could lead to dehydration, electrolyte imbalances, and potentially acute kidney injury. This is particularly relevant during Saxenda's dose escalation phase, when side effects are most pronounced. Patients experiencing severe vomiting or diarrhoea should seek medical advice promptly.
Gallbladder disease is another important consideration. Saxenda has been associated with an increased risk of gallstones and cholecystitis. Symptoms such as right upper quadrant pain, fever, or jaundice should prompt immediate medical attention.
Saxenda should not be used during pregnancy, and should be discontinued if pregnancy occurs or is planned. Women using Saxenda should discuss family planning and consider effective contraception. The lack of comprehensive safety data for berberine supplements means that unexpected interactions or adverse effects cannot be ruled out, emphasising the importance of medical supervision.
Clinical Evidence and Expert Guidance
The clinical evidence base for combining berberine with Saxenda is notably absent. No published randomised controlled trials or observational studies have specifically investigated the safety, efficacy, or interaction profile of this combination. Saxenda's clinical development programme, which led to its MHRA approval, did not include berberine as a concomitant therapy. Consequently, healthcare professionals must rely on theoretical considerations and extrapolation from the known pharmacology of each substance.
Research on berberine as a standalone intervention has shown mixed results. Some meta-analyses suggest modest benefits for glycaemic control and lipid profiles in individuals with metabolic syndrome or type 2 diabetes. However, the quality of evidence is generally low to moderate, with limitations including small sample sizes, heterogeneous study designs, and variable berberine preparations. The European Medicines Agency (EMA) and NICE have not endorsed berberine for any therapeutic indication, and it remains unlicensed as a medicine in the UK.
NICE Technology Appraisal 664 recommends liraglutide for managing overweight and obesity as an adjunct to a reduced-calorie diet and increased physical activity. Under NHS criteria, Saxenda is typically prescribed through specialist weight management services for adults who have a BMI of at least 35 kg/m² (or at least 32.5 kg/m² for certain ethnic groups) with prediabetes or type 2 diabetes, hypertension, dyslipidaemia or obstructive sleep apnoea, and have a high risk of cardiovascular disease. Treatment is usually limited to a maximum of 2 years. The guidance does not address the use of dietary supplements alongside licensed weight management medications.
Healthcare professionals generally advise caution when combining prescription medications with dietary supplements. The Medicines and Healthcare products Regulatory Agency (MHRA) encourages patients to inform their healthcare providers about all supplements they are taking, as interactions can occur even with 'natural' products. The unpredictable quality and composition of berberine supplements available in the UK market further complicates risk assessment. Expert consensus would typically favour using evidence-based, licensed treatments with established safety profiles rather than unproven combinations.
When to Consult Your Healthcare Provider
Seek immediate medical attention if you experience any of the following whilst taking berberine and Saxenda together:
-
Severe hypoglycaemia: confusion, loss of consciousness, inability to treat yourself
-
Severe gastrointestinal symptoms: persistent vomiting, severe diarrhoea lasting more than 24 hours, signs of dehydration (reduced urination, extreme thirst, dry mouth)
-
Allergic reactions: rash, itching, swelling of the face or throat, difficulty breathing
-
Symptoms of pancreatitis: severe, persistent abdominal pain that may radiate to the back, often accompanied by nausea and vomiting
-
Gallbladder problems: severe pain in the upper right side of your abdomen, fever, yellowing of the skin or eyes (jaundice)
-
Severe cardiovascular symptoms: chest pain, severe palpitations, fainting
For mild hypoglycaemia symptoms (trembling, sweating, hunger, dizziness), take a fast-acting carbohydrate (e.g., glucose tablets, fruit juice), check your blood glucose if possible, and seek medical help if symptoms don't improve or recur.
Routine consultation with your GP or prescribing clinician is essential before starting berberine if you are already taking Saxenda, or vice versa. This discussion should cover your complete medication list, including all prescription drugs, over-the-counter medicines, and dietary supplements. Your healthcare provider can assess your individual risk factors, including kidney and liver function, diabetes status, and cardiovascular health.
Regular monitoring may be recommended if you proceed with this combination under medical supervision. This might include periodic blood glucose checks (especially if you have diabetes), assessment of gastrointestinal tolerability, weight monitoring, and evaluation of treatment effectiveness. Your healthcare provider may also wish to review liver and kidney function tests, as both substances are processed through these organs.
If you are considering berberine supplementation whilst taking Saxenda, discuss your motivations with your healthcare team. They can help you evaluate whether additional interventions are necessary or whether optimising your current treatment plan, dietary habits, and physical activity levels might achieve your goals more safely. Remember that weight management is most successful when it combines evidence-based medical treatment with sustainable lifestyle modifications, all coordinated under professional guidance.
If you experience any suspected side effects from either substance, report them to the MHRA through the Yellow Card scheme.
Frequently Asked Questions
Is it safe to take berberine with Saxenda?
There is no official UK guidance on this combination, and safety cannot be assured without medical supervision. Both substances affect glucose metabolism and may cause gastrointestinal side effects, so you should consult your GP or prescribing clinician before combining them.
What are the main risks of combining berberine and Saxenda?
The primary concerns include additive effects on blood glucose (potentially increasing hypoglycaemia risk), intensified gastrointestinal side effects such as nausea and diarrhoea, and unpredictable interactions due to variable quality of berberine supplements.
Should I tell my doctor if I'm taking berberine with Saxenda?
Yes, you must inform your healthcare provider about all supplements and medications you are taking, including berberine. This allows proper assessment of potential interactions and appropriate monitoring of your treatment.
The health-related content published on this site is based on credible scientific sources and is periodically reviewed to ensure accuracy and relevance. Although we aim to reflect the most current medical knowledge, the material is meant for general education and awareness only.
The information on this site is not a substitute for professional medical advice. For any health concerns, please speak with a qualified medical professional. By using this information, you acknowledge responsibility for any decisions made and understand we are not liable for any consequences that may result.
Heading 1
Heading 2
Heading 3
Heading 4
Heading 5
Heading 6
Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut aliquip ex ea commodo consequat. Duis aute irure dolor in reprehenderit in voluptate velit esse cillum dolore eu fugiat nulla pariatur.
Block quote
Ordered list
- Item 1
- Item 2
- Item 3
Unordered list
- Item A
- Item B
- Item C
Bold text
Emphasis
Superscript
Subscript










