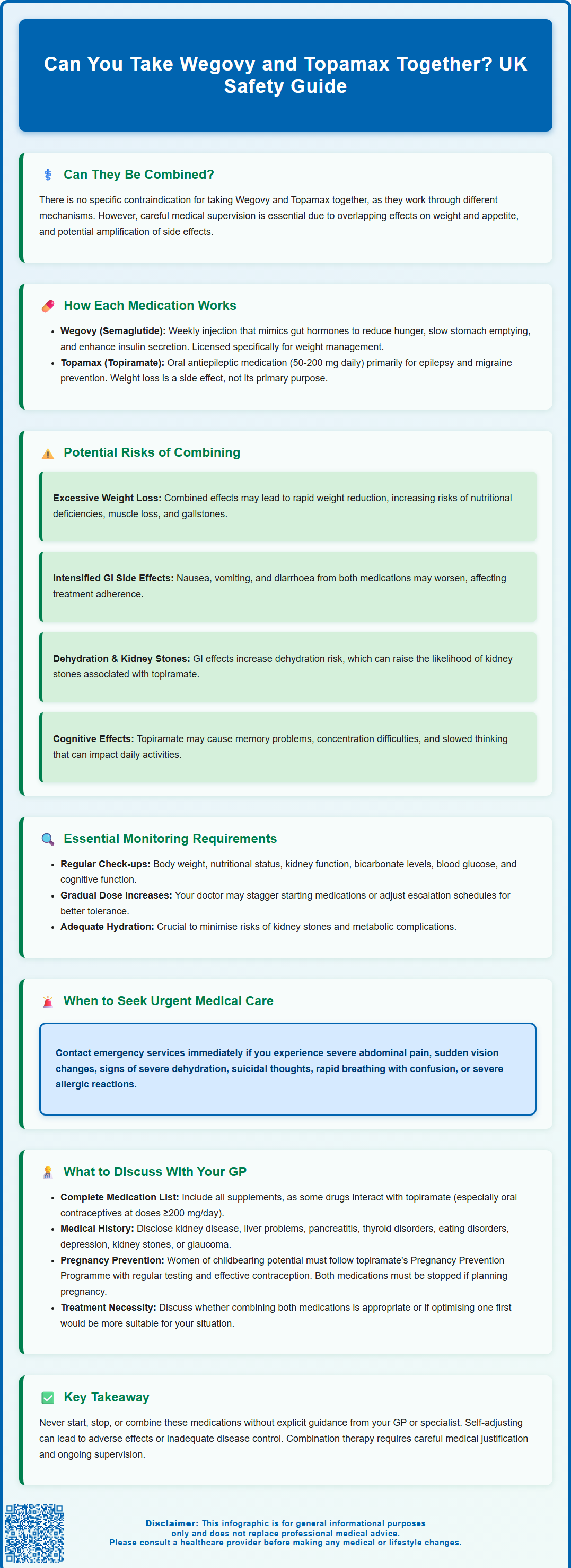
Can you take Wegovy and Topamax together? Many patients prescribed Wegovy (semaglutide) for weight management wonder whether it is safe to combine with Topamax (topiramate), particularly if already using topiramate for migraine prevention or epilepsy. There is no specific contraindication in UK product information for taking these medications together, as they work through different mechanisms. However, combining them requires careful medical supervision due to overlapping effects on weight and appetite, plus potential side effects that may be amplified. This article explains how these medicines work, their potential interactions, and essential safety considerations. Always consult your GP or specialist before starting, stopping, or combining these medications.
Summary: Wegovy (semaglutide) and Topamax (topiramate) can be taken together as there is no specific contraindication, but combination therapy requires careful medical supervision due to overlapping effects on weight and potential additive side effects.
- Wegovy is a GLP-1 receptor agonist licensed for chronic weight management, whilst Topamax is an antiepileptic drug indicated for epilepsy and migraine prophylaxis.
- No direct pharmacokinetic interaction exists between semaglutide and topiramate as they are metabolised through different pathways.
- Both medications can cause weight loss and gastrointestinal side effects, which may be additive when used together.
- Regular monitoring of weight, kidney function, serum bicarbonate, and nutritional status is essential during combination therapy.
- Both medications are contraindicated or require strict precautions in pregnancy, with topiramate now requiring a Pregnancy Prevention Programme for women of childbearing potential.
- Patients should never start, stop, or adjust doses without explicit guidance from their GP or specialist prescriber.
Table of Contents
Can You Take Wegovy and Topamax Together?
Many patients prescribed Wegovy (semaglutide) for weight management wonder whether it is safe to take alongside Topamax (topiramate), particularly if they are already using topiramate for migraine prevention or epilepsy. The short answer is that there is no specific contraindication listed in UK product information for taking these two medications together, and they work through different mechanisms in the body. However, combining them requires careful medical supervision due to overlapping effects on weight and appetite, as well as potential side effects that may be amplified when used concurrently.
Wegovy is a glucagon-like peptide-1 (GLP-1) receptor agonist licensed for chronic weight management in adults and adolescents aged 12 years and older with obesity or overweight with weight-related comorbidities. Topamax, on the other hand, is an antiepileptic drug (AED) primarily indicated for epilepsy and migraine prophylaxis. While topiramate can cause weight loss as a side effect, using it primarily for weight management is considered off-label in the UK and should only be considered under specialist care when indicated for its licensed uses.
It is essential that you do not start, stop, or combine these medications without explicit guidance from your GP or specialist prescriber. Self-medicating or adjusting doses independently can lead to adverse effects, inadequate disease control, or dangerous drug interactions. This article provides educational information to help you understand how these medications work, their potential interactions, and the key safety considerations, but it should not replace personalised medical advice tailored to your individual health circumstances.
How Wegovy and Topamax Work in the Body
Understanding the distinct pharmacological actions of Wegovy and Topamax is crucial to appreciating why their combination requires clinical oversight.
Wegovy (semaglutide) is a long-acting GLP-1 receptor agonist administered as a once-weekly subcutaneous injection. It mimics the action of the naturally occurring incretin hormone GLP-1, which is released from the gut in response to food intake. Semaglutide works by:
-
Enhancing insulin secretion in a glucose-dependent manner, which helps regulate blood sugar levels
-
Suppressing glucagon release, reducing hepatic glucose output
-
Slowing gastric emptying, which prolongs satiety and reduces appetite
-
Acting on appetite centres in the brain, particularly the hypothalamus, to reduce hunger and food intake
These combined effects led to significant weight loss in clinical trials (STEP programme), typically in the range of 10–15% of body weight over 68 weeks, though individual results vary considerably. Wegovy is licensed specifically for weight management in the UK for adults and adolescents (≥12 years) with specific BMI criteria, and is not indicated for type 2 diabetes treatment, though its sister product Ozempic (also semaglutide) is used for glycaemic control.
Topamax (topiramate) is an antiepileptic drug with a more complex and not fully understood mechanism of action. It is believed to work through multiple pathways, including:
-
Blocking voltage-gated sodium channels, reducing neuronal excitability
-
Enhancing GABA (gamma-aminobutyric acid) activity, an inhibitory neurotransmitter
-
Antagonising glutamate receptors, further dampening excitatory neurotransmission
-
Inhibiting carbonic anhydrase enzymes, though the clinical significance of this effect is uncertain
While topiramate's primary indications are epilepsy and migraine prophylaxis, it has been observed to cause weight loss as a side effect, likely through appetite suppression and altered taste perception. Topiramate is typically taken orally once or twice daily, with doses for migraine prevention usually between 50–100 mg daily (maximum 200 mg daily), while epilepsy treatment may require higher doses.
Potential Interactions Between Wegovy and Topamax
From a strict pharmacokinetic perspective, there is no direct drug-drug interaction between semaglutide and topiramate. They are metabolised and eliminated through different pathways: semaglutide is broken down by proteolytic enzymes (similar to endogenous proteins) and does not rely on cytochrome P450 liver enzymes, whilst topiramate is partially metabolised hepatically but largely excreted unchanged in urine. Neither medication significantly affects the blood levels or clearance of the other.
However, there are some important considerations:
- Delayed gastric emptying: Semaglutide significantly slows gastric emptying, which could potentially delay the absorption of oral medications like topiramate. While this is usually not clinically significant for most medications, it's worth noting, particularly when initiating treatment.
Pharmacodynamic interactions—where two drugs produce overlapping or additive effects—are more relevant when considering this combination:
-
Additive weight loss effects: Both medications can cause significant weight reduction. Whilst this may seem beneficial for patients with obesity, excessive or rapid weight loss can lead to nutritional deficiencies, muscle wasting, gallstone formation, and metabolic disturbances. Close monitoring of weight trajectory and nutritional status is essential.
-
Gastrointestinal side effects: Wegovy commonly causes nausea, vomiting, diarrhoea, and constipation, particularly during dose escalation. Topiramate can also cause gastrointestinal upset, though typically less pronounced. When combined, these effects may be more troublesome and could affect medication adherence or quality of life.
-
Dehydration risk: GI side effects from semaglutide may increase the risk of dehydration, which can in turn increase the risk of kidney stones with topiramate. Maintaining adequate hydration is particularly important with this combination.
-
Cognitive effects: Topiramate is well known for causing cognitive side effects, including difficulty with concentration, memory problems, and slowed thinking (sometimes referred to colloquially as "Dopamax"). Whilst semaglutide does not typically cause cognitive impairment, patients taking both medications should be aware of these potential effects, which may impact daily functioning, work performance, or driving ability.
-
Metabolic acidosis risk: Topiramate can cause metabolic acidosis (elevated blood acidity) due to its carbonic anhydrase inhibitory effects. This is generally mild but can be clinically significant in some patients. There is no evidence that semaglutide affects acid-base balance, but patients on combination therapy should have their bicarbonate levels monitored, particularly if they have kidney disease or are taking other medications that affect acid-base status.
Safety Considerations When Combining These Medications
If your healthcare provider determines that taking Wegovy and Topamax together is appropriate for your clinical situation, several safety considerations should guide your treatment:
Monitoring and follow-up: Regular clinical review is essential. Your GP or specialist should monitor:
-
Body weight and body composition to ensure weight loss is appropriate and not excessive
-
Nutritional status, including vitamin and mineral levels, particularly if gastrointestinal side effects are prominent
-
Kidney function (serum creatinine, eGFR) – topiramate requires dose adjustment when creatinine clearance is <70 mL/min; semaglutide does not require dose adjustment but caution is needed if GI symptoms risk dehydration
-
Serum bicarbonate levels to detect metabolic acidosis, particularly in the first few months of topiramate therapy
-
Blood glucose levels if you have diabetes or prediabetes, as semaglutide affects glycaemic control
-
Cognitive function and mood, given topiramate's neuropsychiatric effects
Dose titration and timing: Both medications typically require gradual dose escalation to minimise side effects. Wegovy follows a structured dose-escalation schedule over 16–20 weeks, whilst topiramate is usually increased slowly over several weeks. If starting both medications, your prescriber may stagger the initiation or adjust the titration schedule to better tolerate side effects.
Contraindications and cautions: Be aware of specific situations where one or both medications should be avoided or used with extreme caution:
-
Pregnancy: Both medications should be avoided in pregnancy. Topiramate is now contraindicated for migraine prophylaxis in pregnancy and in women of childbearing potential unless a Pregnancy Prevention Programme (PPP) is followed. For epilepsy, topiramate should only be used in pregnancy if no suitable alternatives exist, with specialist oversight. Semaglutide should be discontinued at least 2 months before planned pregnancy. Effective contraception is essential.
-
History of pancreatitis: Semaglutide should be used with caution, and patients should be counselled on symptoms of pancreatitis (severe, persistent abdominal pain).
-
Gallbladder disease: GLP-1 receptor agonists like semaglutide may increase the risk of gallstones and gallbladder inflammation.
-
Kidney stones: Topiramate increases the risk of nephrolithiasis; adequate hydration is important, especially given the potential for dehydration with semaglutide's GI effects.
-
Glaucoma: Topiramate can rarely cause acute angle-closure glaucoma, typically within the first month of treatment.
Medication discontinuation: If topiramate needs to be stopped, it should be gradually tapered rather than abruptly discontinued to reduce the risk of seizures, even in patients without epilepsy.
When to seek urgent medical attention: Contact your GP or seek emergency care if you experience:
-
Severe, persistent abdominal pain (possible pancreatitis)
-
Right upper quadrant pain, fever, or jaundice (possible gallbladder disease)
-
Sudden vision changes or eye pain (possible acute glaucoma)
-
Signs of severe dehydration (dizziness, reduced urination, confusion)
-
Suicidal thoughts or severe mood changes
-
Symptoms of metabolic acidosis (rapid breathing, confusion, fatigue)
-
Severe allergic reactions (rash, swelling, difficulty breathing)
If you experience any suspected side effects, you can report them through the MHRA Yellow Card scheme.
What to Discuss With Your GP or Prescriber
Before combining Wegovy and Topamax, or if you are already taking both medications, it is important to have an open and thorough discussion with your healthcare provider. Come prepared with information about your complete medical history and current medications to facilitate shared decision-making.
Key topics to cover include:
-
Your complete medication list: Include all prescription medications, over-the-counter drugs, herbal supplements, and vitamins. Some medications may interact with topiramate (such as oral contraceptives, which may be less effective particularly at doses ≥200 mg/day) or affect the tolerability of semaglutide.
-
Your medical history: Discuss any history of kidney disease, liver problems, pancreatitis, thyroid disorders, eating disorders, depression or suicidal ideation, kidney stones, or glaucoma. These conditions may influence whether combination therapy is appropriate.
-
Your treatment goals: Be clear about why you are taking or considering each medication. If both are being used primarily for weight loss, your prescriber may discuss whether combination therapy is necessary or whether optimising one medication first would be more appropriate. NICE guidance (TA875) supports the use of semaglutide for weight management in specific circumstances through specialist weight management services, but combination pharmacotherapy requires careful justification.
-
Previous medication experiences: If you have previously tried either medication, discuss your response and any side effects experienced. This information helps predict tolerability of combination therapy.
-
Contraception and pregnancy plans: If you are a woman of childbearing potential, discuss contraception options. For topiramate, the MHRA now requires a Pregnancy Prevention Programme including pregnancy testing before initiation, highly effective contraception throughout treatment, annual specialist review, and a patient guide with risk acknowledgement. If you are planning pregnancy, you will need to discontinue both medications with appropriate timing.
-
Monitoring plan: Clarify how often you will need follow-up appointments, which blood tests or other investigations will be required, and what symptoms should prompt you to seek earlier review.
-
Cost and access considerations: Wegovy is currently available through the NHS in limited circumstances according to NICE guidance and may require private prescription for many patients. Discuss the financial implications and sustainability of long-term treatment.
Questions you might ask your prescriber:
-
What are the specific benefits and risks of taking these medications together in my situation?
-
Are there alternative treatment options that might be safer or more effective?
-
How will we monitor for potential side effects or complications?
-
What should I do if I experience troublesome side effects?
-
How long will I need to take these medications?
-
What happens if I need to stop one or both medications?
Remember that your healthcare provider's recommendations should be based on a comprehensive assessment of your individual circumstances, including your medical history, current health status, treatment goals, and personal preferences. Never adjust your medication regimen without professional guidance, and maintain open communication with your healthcare team throughout your treatment journey.
Scientific References
Frequently Asked Questions
Is there a drug interaction between Wegovy and Topamax?
There is no direct pharmacokinetic interaction between Wegovy (semaglutide) and Topamax (topiramate), as they are metabolised through different pathways. However, they may have additive effects on weight loss and gastrointestinal side effects, requiring careful medical supervision when used together.
What monitoring is needed when taking Wegovy and Topamax together?
Regular monitoring should include body weight and nutritional status, kidney function (serum creatinine, eGFR), serum bicarbonate levels to detect metabolic acidosis, blood glucose if diabetic, and assessment of cognitive function and mood. Your GP or specialist will determine the appropriate monitoring schedule based on your individual circumstances.
Can I take Wegovy and Topamax together if I'm planning pregnancy?
No, both medications should be avoided in pregnancy. Semaglutide should be discontinued at least 2 months before planned pregnancy, whilst topiramate is now contraindicated for migraine prophylaxis in women of childbearing potential unless a Pregnancy Prevention Programme is followed with highly effective contraception. Discuss pregnancy plans with your GP or specialist before starting or continuing these medications.
The health-related content published on this site is based on credible scientific sources and is periodically reviewed to ensure accuracy and relevance. Although we aim to reflect the most current medical knowledge, the material is meant for general education and awareness only.
The information on this site is not a substitute for professional medical advice. For any health concerns, please speak with a qualified medical professional. By using this information, you acknowledge responsibility for any decisions made and understand we are not liable for any consequences that may result.
Heading 1
Heading 2
Heading 3
Heading 4
Heading 5
Heading 6
Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut aliquip ex ea commodo consequat. Duis aute irure dolor in reprehenderit in voluptate velit esse cillum dolore eu fugiat nulla pariatur.
Block quote
Ordered list
- Item 1
- Item 2
- Item 3
Unordered list
- Item A
- Item B
- Item C
Bold text
Emphasis
Superscript
Subscript










